
Educational content provided by Alimera Sciences.
There are a variety of treatment options for patients with diabetic macular edema (DME); options that can include mono-therapeutic or combination therapeutic approaches using laser photocoagulation, anti-vascular endothelial growth factors (anti-VEGFs), and corticosteroids. Switching between treatment regimens is often a consequence of the previous treatment eliciting an insufficient therapeutic response, and therein lies the problem. How does one decide when a response is insufficient? Is there a universal set of criteria that can be followed, on which to base clinical decisions? Currently, there are several definitions of treatment insufficiency for DME. These include (but not exclusively):
- Persisting intraretinal cysts with a reduction in central retinal thickness (CRT) of less than 20 percent1
- A residual central subfield thickness per optical coherence tomography (OCT) of more than 325 µm1
- Less than a 130 µm reduction in macular thickness with a fewer than five to seven letter gain in vision after 3–4 months of anti-VEGF treatment2
- Or just simply a change in best-corrected visual acuity (BCVA) <5 letters.3
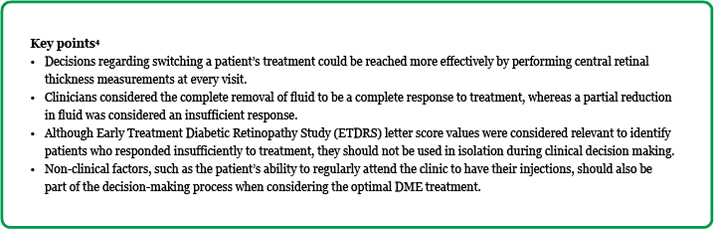
Insufficient response based on retinal thickness When considering how to define an insufficient response based on retinal thickness, most clinicians considered the percentage reduction from baseline to be more important than the absolute reduction, and felt that any reduction in CRT less than 20 percent from the original baseline values would warrant investigating an alternative therapeutic approach. Other clinicians, however, used empirical reduction thresholds of less than 50 µm, while some preferred to rely on their own clinical experience. Despite the different suggestions, it was generally felt that decisions regarding treatment switching could be reached more effectively by performing CRT measurements at every visit. Although CRT measurements can fluctuate markedly in some patients at different visits, regular reporting allows the detection of trends and avoids treatment decisions based on single time points. This type of analysis can help to determine whether improvements are restricted to early stages of treatment or whether they are more progressive and, thus, switching treatment may be considered for those individuals where improvements are not progressing at a sufficient rate, or where the response appears muted.4
Insufficient response based on retinal edema When considering how to define an insufficient response based on retinal edema, clinicians considered the complete removal of fluid to be a complete response, whereas a partial reduction in fluid was considered an insufficient response. It was also deemed important to undertake comparison mapping to areas outside the subfoveal area as edema improvements may occur in these areas first.4
Insufficient response based on visual acuity outcomes In order to determine whether a patient with DME responded insufficiently to treatment, visual acuity outcomes, measured as Early Treatment Diabetic Retinopathy Study (ETDRS) letter score values were also considered to be relevant. An ETDRS letter score improvement greater than 15 letters was deemed an excellent response. An improvement of five to ten or five–15 letters was considered a moderate response whereas, due to the inherent variability of measuring scores between zero and five, any such results were deemed an insufficient response to treatment. However, since visual acuity can fluctuate in diabetic patients, the clinicians concluded that it was not adequate to use this criterion in isolation to gauge therapeutic benefits. Rather, it should be considered in combination with other factors, such as CRT improvements and the multifactorial nature of diabetic retinopathy, during the decision making process. For example, individuals with CRT improvements less than 20 percent and with no perceived improvements in visual acuity should be considered for alternative treatment strategies.4 Similarly, alternative treatment strategies might also be required given the complex nature of diabetic retinopathy. For example, when the mean BCVA change was examined over 36 months in the RIDE and RISE trials in patients with DME treated with ranibizumab, the greatest change from baseline occurred within the first three months of treatment.7 Interestingly, this rise was not observed when ranibizumab was administered to the sham group at Month 24.8 Pooled results from the RIDE and RISE trials showed that the percentage gain of ≥15 letters, measured 12 months after 0.3 mg ranibizumab administration was 32.4 percent in the treatment group compared to only 7.3 percent when ranibizumab was administered to the sham group (eligible to ranibizumab treatment after Month 24). This reduction in response in the sham group might be related to the delay in treatment, or could be attributed to the accepted multifactorial nature and natural history of diabetic retinopathy.8,9
Non-clinical factors to be considered when evaluating DME treatment response—looking beyond clinical outcomes In addition to the factors mentioned above, non-clinical factors were also considered to be important when evaluating the effectiveness of any prescribed DME treatment. Poor treatment effectiveness might be due to patients being unable and/or unwilling to comply with a course of treatment. Some therapeutic options, such as anti-VEGF treatment, initially rely on monthly intravitreal injections. These treatment requirements can impose a large burden on patients, as well as factors such as lifestyle, age, job, lack of a support network, and the potential for having to attend other clinics due to co-morbidities. Even though it would be clinically beneficial for some individuals to receive monthly treatments, it might actually be more pragmatic in these cases to examine other treatment options that are less cumbersome, and avoid the potential for suboptimal efficacy due to non-adherence.4
Conclusion Multiple clinical criteria should be considered when determining whether a DME treatment strategy has resulted in a sufficient therapeutic response. In addition, non-clinical factors are also important when evaluating treatment effectiveness. Treatment decisions should take a holistic view of an individual patient’s clinical situation and his/her preferences, and allow the clinical professional the flexibility to provide experience-based treatment, specific to that patient, without being restricted by rigid clinical criteria. Thus, although empirical measurements are important, they should be used as a guide rather than a mandatory decision tool when selecting optimal therapeutic strategies for treating DME.4
Look out for DME content developed by Alimera Sciences on this website throughout 2015 and 2016. We hope it supports your knowledge of DME, and if you would like to contribute material for publication, please send your materials to dmecontenthub@hayward.co.uk, we’d be very pleased to consider your contributions.

REFERENCES
- J Hanhart, I. Chowers, “Evaluation of the response to ranibizumab therapy following bevacizumab treatment failure in eyes with diabetic macular edema”, Case Rep Ophthalmol, 6, 44–50 (2015). PMID: 25802504. (doi: 10.1159/000375230).
- F Quhill, “Real-world experience of fluocinolone acetonide (0.2 µg/day) intravitreal implant in the treatment of diabetic macular oedema”, European Ophthalmic Review, 9(1), 42–46 (2015).
- A Erginay et al., ARVO 2015 annual meeting Abstracts, “Real-life experience following the usage of 0.2 mg/day Fluocinolone Acetonide implant in diabetic macular edema patients”, (2015). Available at: http://www.arvo.org/webs/am2015/abstract/240.pdf. Accessed September 3, 2015.
- Data on File. Alimera Sciences.
- The free dictionary by Farlex, “Definition of optical coherence tomography”, (2015). Available at: http://medical-dictionary.thefreedictionary.com/Optical+coherence+tomography. Accessed September 3, 2015.
- The free dictionary by Farlex, “Definition of subfoveal area”, (2015). Available at: http://medical-dictionary.thefreedictionary.com/subfoveal. Accessed September 3, 2015.
- QD Nguyen et al. on behalf of the RISE and RIDE Research Group, “Ranibizumab for diabetic macular edema. Results from 2 phase III randomized trials: RISE and RIDE”, Ophthalmology, 119, 789–801 (2012). PMID: 22330964.
- DM Brown et al., “Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE”, Ophthalmol, 120, 44–50 (2013). PMID: 23706949.
- N Bhagat et al., “Diabetic macular edema: pathogenesis and treatment”, Surv Ophthalmol, 54(1), 1–32 (2009). PMID: 19171208.
Founded in 2003, Alimera Sciences researches and develops innovative vision-improving treatments for chronic retinal diseases, such as diabetic macular edema (DME), dry age-related macular degeneration (AMD), and retinal vein occlusion. In 2015, Alimera Sciences partnered with The Ophthalmologist to facilitate the publication of independently created educational content surrounding DME, a serious retinal complication associated with diabetes, which is increasing in incidence with the increasing prevalence of diabetes worldwide. Published content will include articles ranging from basic science and disease processes to overviews of clinical data, different surgical procedures, comparisons of treatment options, and practical advice for managing diabetic patients. With a commitment to honesty, integrity, responsibility, candor, and trust, Alimera Sciences intend to provide educationally focused content to healthcare professionals across a wide range of topics in DME in order to both increase disease awareness and understanding, and to help improve patient outcomes. UK-ILV-MMM-0355 Date of preparation: August 2015 enquiries@alimerasciences.com

