
Advertisement feature; ILUVIEN associated with significant improvements in visual gain and central retinal thickness within three months, with good tolerability, in a retrospective, observational UK-based study
Funded by Alimera Sciences. Contains promotional information. Please click here to view the prescribing information and adverse event reporting information.
An abstract presented at the 2015 Canadian Ophthalmological Society (COS) Annual Meeting and Exhibition (18–21 June, 2015; Victoria, British Columbia) provides real life data examples of how chronic diabetic macular edema (DME), which was previously unresponsive to available therapies, responds positively to treatment with ILUVIEN® (fluocinolone acetonide [FAc]) intravitreal implant (Alimera Sciences) with improvements in visual gain (VG) and central retinal thickness (CRT) within three months, with no adverse events reported.1,2
The UK-based, retrospective, observational study – authored by Dr Ibrahim Elaroud and colleagues – included 23 patients (15 males and eight females) with chronic DME, which had persisted despite therapy with available treatments.2

In the Elaroud et al study, patients with chronic DME received a single treatment with ILUVIEN between January and August 2014, and were followed for three months. Three distinct responses to ILUVIEN treatment were observed: both a structural (reduction in CRT) and functional (increase in VG) improvement in chronic DME, a structural improvement only, or no structural or functional improvement (Figure 1).2 For full long-term benefits of treatment with ILUVIEN, a longer observation period is required.
Figure 1. Response to treatment with ILUVIEN within 3 months among patients with chronic DME.2
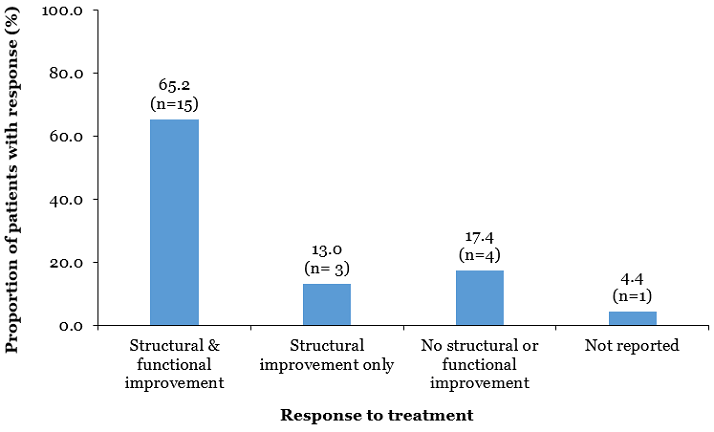
Treatment with ILUVIEN led to rapid increases in VG and reductions in CRT for the majority of patients. Overall in the three month study, 65% of patients (15/23) showed both a structural and functional improvement in chronic DME, with an average Early Treatment of Diabetic Retinopathy Study (ETDRS)‐letters gain of 7.8 (range 3–25) and a mean reduction in CRT of 205.5 μm. An improvement in ETDRS score of 10 letters occurred in five patients, of five to 10 letters in seven patients, and three to four letters in two patients. An improvement in CRT only was observed in 13% of patients (3/23), with an average reduction in CRT of 311 μm. No improvement in CRT or VG occurred in four patients (17.4%).2
ILUVIEN was also shown to be well-tolerated during the 3 months of follow-up. Prior to the study, five patients had received treatment for intraocular hypertension. However, following ILUVIEN administration, no spikes in intraocular pressure were observed, including the patients who had been treated for intraocular hypertension previously. No patient experienced retinal detachment or endophthalmitis, although two patients complained of floaters, which settled within two weeks.2
These data demonstrate that ILUVIEN can provide rapid structural and visual improvements and is well-tolerated in patients with chronic DME.2 It is therefore suggested that patients with chronic DME should receive an early single injection of ILUVIEN to optimize outcomes, rather than treating with multiple intermittent therapies.
Look out for DME content developed by Alimera Sciences on this website throughout 2015. We hope it supports your knowledge of DME and ILUVIEN, and if you would like to contribute material for publication, please send your materials to dmecontenthub@hayward.co.uk, we’d be very pleased to consider your contributions.
REFERENCES 1. Canadian Ophthalmological Society (COS) 2015 Annual Meeting and Exhibition website. Available at www.cos-sco.ca/victoria2015 (Accessed June 2015) 2. I Elaroud et al., “Fluocinolone acetate role in treatment of chronic diabetic macular oedema. A 3-months results of a multi-centred retrospective UK observational study”, Abstract presented at the 2015 Canadian Ophthalmological Society (COS) Annual Meeting and Exhibition Victoria, British Columbia, June 18–21, (2015). 3. The Royal College of Ophthalmologists Diabetic Retinopathy Guidelines. December 2012. Available at www.rcophth.ac.uk/wp-content/uploads/2014/12/2013-SCI-301-FINAL-DR-GUIDELINES-DEC-2012-updated-July-2013.pdf (Accessed June 2015) 4. P Campochiaro et al., “Long-term Benefit of Sustained-Delivery Fluocinolone Acetonide Vitreous Inserts for Diabetic Macular Edema”, Ophthalmology, 118, 626–635 (2011). PMID: 21459216. 5. L Downey et al., “Exploratory analyses of long-term visual outcomes based on baseline vision in patients with chronic and non-chronic diabetic macular oedema (DMO) treated with fluocinolone acetonide (FAc)”, Poster No. 221, Poster presented at the Royal College of Ophthalmologists Annual Congress, Liverpool, UK, 19–21 May, (2015).
UK-ILV-MMM-0271
Date of preparation: June 2015
Founded in 2003, Alimera Sciences researches and develops innovative vision-improving treatments for chronic retinal disease. Alimera Sciences has developed and licensed ILUVIEN®, an intravitreal implant of 190 micrograms fluocinolone acetonide, for the treatment of vision impairment associated with chronic diabetic macular edema (DME), considered insufficiently responsive to available therapies.a ILUVIEN is the first DME treatment to deliver up to 36 months of continuous, low-dose corticosteroid by single injection.b In 2015, Alimera Sciences has partnered with The Ophthalmologist to facilitate the publication of independently created content on ILUVIEN and DME. Content will range from conference reports, case studies, and literature reviews to video interviews, presentations, and practical information surrounding the use and benefits of ILUVIEN. The word Alimera derives loosely from the Greek, to mean “day of truth”.c With a commitment to honesty, integrity, responsibility, candor, and trust, Alimera Sciences intend that this promotional information accurately and fairly represents the current state of knowledge of ILUVIEN and DME, and is useful to all healthcare professionals involved in DME and its treatment. a. ILUVIEN SPC. 2013 Available at: www.medicines.org.uk/emc/medicine/27636 (Accessed March 2015) b. Alimera Sciences. Available at www.alimerasciences.com (Accessed March 2015) c. Retina Today. 2011. Available at: http://retinatoday.com/pdfs/0111RT_Wall.Street.pdf (Accessed March 2015) UK-ILV-MMM-0167 Date of preparation: March 2015 www.alimerasciences.com enquiries@alimerasciences.com

