
Advertisement feature; Vera Schmit-Eilenberger publishes efficacy data on ILUVIEN use in chronic DME cases
Funded by Alimera Sciences. Contains promotional information. Please click here to view the prescribing information and adverse event reporting information.
A real-world evaluation of ILUVIEN® (fluocinolone acetonide [FAc]) intravitreal implant (Alimera Sciences) has demonstrated rapid and sustained improvements in best corrected visual acuity (BCVA) and central foveal thickness (CFT) following a single treatment with the drug.1 The case series included a range of patients with chronic diabetic macular edema (DME) insufficiently responsive to other therapies. According to the author of the study, Dr Vera Schmit-Eilenberger,
Dr Schmit-Eilenberger goes on to say that “for the future we do hope that sustained intravitreal devices will be the standard for everybody who might benefit.”2
The retrospective registry study followed 10 patients (15 eyes) treated with a single intravitreal injection of 0.2 µg per day FAc between May 2013 and March 2014. Overall, nine patients were pseudophakic at baseline and one patient had bilateral cataract, which was removed 5–6 weeks after ILUVIEN treatment. Seven eyes in five patients had previously undergone vitrectomy. All patients had received previous treatment with anti-vascular endothelial growth factor (anti-VEGF) drugs (ranibizumab 0.5 mg [2–7 injections] or bevacizumab 1.25 mg [1–15 injections]) and corticosteroids (triamcinolone acetonide 4–25 mg [1–7 injections] or dexamethasone implant 700 µg [1–2 injections]), but had had an insufficient response.1
The main outcome measure of the study was BCVA, which was assessed pre and post-treatment on day one and assessed at each follow-up visit. Secondary outcomes measures included CFT and intraocular pressure (IOP). At baseline, BCVA ranged from 0.1 to 0.6, CFT was 291–729 µm, and IOP was 12–19 mmHg.1 BCVA improved at the last follow-up visit, compared with baseline, in 10/15 eyes (66.7 percent). The mean gain in BCVA from baseline was 0.19 (range –0.1 to +0.75). BCVA was unchanged compared with baseline in three eyes and decreased slightly (–0.1 and –0.05) in two eyes. Gains in BCVA were independent of any previous treatment.1
ILUVIEN also led to reductions in CFT; at baseline, CFT ranged from 291 to 729 µm (in 10 of the 15 eyes). Following ILUVIEN administration, CFT decreased in all 10 eyes by an average of 206.3 μm.1 IOP increased by >7 mmHg in 3/15 eyes, however these increases were managed successfully in all three cases. The mean change in IOP was 4.1 ± 5.8 mmHg from a mean baseline value of 15.5 ± 2.3 mmHg.1
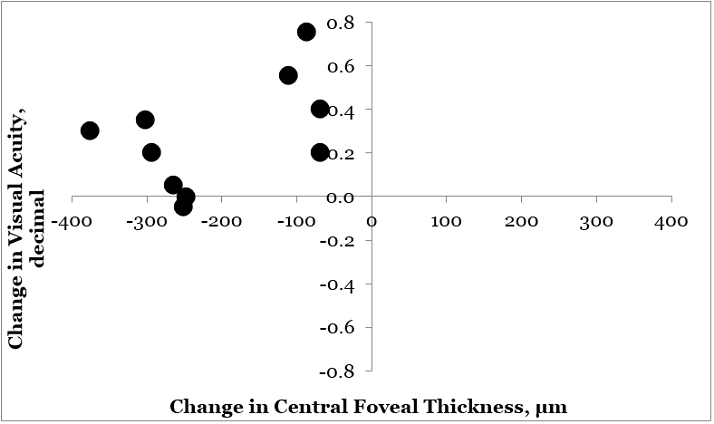
Figure 1. Comparison of Central Foveal Thickness versus Visual Acuity - individual plots (n=10). Central foveal thickness plotted against visual acuity for 10 (of 15) eyes in which paired measurements were conducted. The values for visual acuity and central foveal thickness prior to ILUVIEN ranged between 0.10-0.60 and 729-291 µm, respectively. Adapted from V Schmit-Eilenberger. 2015.
Observations from one patient were specifically reported. The patient had previously received treatment with bevacizumab (four injections in the right eye [RE]), ranibizumab (two injections, RE), and bevacizumab (three injections, LE); had undergone vitrectomy; and was bilaterally pseudophakic. BCVA at baseline was 0.25 in the right eye (RE) and 0.2 in the LE, CFT was 541 µm in the RE and 504 µm in the LE, and IOP was 18 mmHg in both eyes. ILUVIEN was injected into both eyes in January 2014.1
Following treatment, BCVA improved within 1–3 weeks: to 0.4 in the RE and 0.3 in the LE. Further improvements in BCVA were observed at 7–9 weeks after ILUVIEN treatment: to 0.6 in the RE and 0.4 in the LE. CFT decreased during follow-up, decreasing by 301 µm by week seven in the RE and by 294 µm at week nine in the LE. IOP increased to 26 mmHg in the RE at week seven and to 25 mmHg in the LE at week nine. Treatment with a fixed combination of dorzolamide/timolol was initiated, which reduced IOP to ≤20 mmHg. Similar responses were seen in patients unresponsive to prior therapies that had a longer follow-up period (up to 37 weeks).1
Key findings Currently there is limited information available on the outcomes of patients treated with FAc under real-life conditions. Data from this study demonstrates that patients who were insufficiently responsive to prior anti-VEGF (at least three injections) and corticosteroid (at least one injection was administered in all but three eyes), experienced sustained improvements in CFT and BCVA (in around 67% of eyes) with a single ILUVIEN injection.1,3 Increases in IOP were managed successfully in all three cases of occurrence.
This study demonstrates that ILUVIEN can be effective, with a favorable risk-benefit profile in patients that are pseudophakic, have undergone vitrectomy, and who have been extensively and unsuccessfully treated previously with anti-VEGF and short-acting corticosteroids.1,2 Marked improvements were seen across the patient cases, see Box 1.
Dr Schmit-Eilenberger suggests that ILUVIEN can result in,
Despite the benefits of ILUVIEN, Dr Schmit-Eilenberger highlights that caution is needed: “BCVA, potential development of cataracts, raised IOP, and the findings in the retina should be monitored on a regular basis, depending on the individual situation.”2
Dr Vera Schmit-Eilenberger is a senior physician at the Department of Ophthalmology, Municipal Hospital Karlsruhe, Karlsruhe, Germany. Her experience includes retinal and cataract surgery; with interests in diabetic macular edema, diabetic macular retinopathy, age-related macular edema and retinal vein occlusion. Look out for DME content developed by Alimera Sciences on this website throughout 2015. We hope it supports your knowledge of DME and ILUVIEN, and if you would like to contribute material for publication, please send your materials to dmecontenthub@hayward.co.uk, we’d be very pleased to consider your contributions.

REFERENCES 1. V Schmit-Eilenberger, “A novel intravitreal fluocinolone acetonide implant (Iluvien®) in the treatment of patients with chronic diabetic macular edema that is insufficiently responsive to other medical treatment options: a case series”, Clinical Ophthalmology, 9, 1–11 (2015). PMID: 25999689. Available at: http://www.dovepress.com/a-novel-intravitreal-fluocinolone-acetonide-implant-iluvienreg-in-the--peer-reviewed-fulltext-article-OPTH 2. Vera Schmit-Eilenberger interview with Hayward Medical Communications. 2015. 3. Personal communication with Vera Schmit-Eilenberger. June 2015. 4. Visual acuity conversion chart
EUR-ILV-MMM-0077 Date of preparation: May 2015
Founded in 2003, Alimera Sciences researches and develops innovative vision-improving treatments for chronic retinal disease. Alimera Sciences has developed and licensed ILUVIEN®, an intravitreal implant of 190 micrograms fluocinolone acetonide, for the treatment of vision impairment associated with chronic diabetic macular edema (DME), considered insufficiently responsive to available therapies.a ILUVIEN is the first DME treatment to deliver up to 36 months of continuous, low-dose corticosteroid by single injection.b In 2015, Alimera Sciences has partnered with The Ophthalmologist to facilitate the publication of independently created content on ILUVIEN and DME. Content will range from conference reports, case studies, and literature reviews to video interviews, presentations, and practical information surrounding the use and benefits of ILUVIEN. The word Alimera derives loosely from the Greek, to mean “day of truth”.c With a commitment to honesty, integrity, responsibility, candor, and trust, Alimera Sciences intend that this promotional information accurately and fairly represents the current state of knowledge of ILUVIEN and DME, and is useful to all healthcare professionals involved in DME and its treatment. a. ILUVIEN SPC. 2013 Available at: www.medicines.org.uk/emc/medicine/27636 (Accessed March 2015) b. Alimera Sciences. Available at www.alimerasciences.com (Accessed March 2015) c. Retina Today. 2011. Available at: http://retinatoday.com/pdfs/0111RT_Wall.Street.pdf (Accessed March 2015) UK-ILV-MMM-0167 Date of preparation: March 2015 www.alimerasciences.com enquiries@alimerasciences.com

