
Advertisement feature; Louise Downey presents exploratory analyses of long-term visual outcomes in patients with chronic and non-chronic DME treated with fluocinolone acetonide
Funded by Alimera Sciences. Contains promotional information. Please click here to view the prescribing information and adverse event reporting information.
A poster presented at the Royal College of Ophthalmologists annual congress 2015 — by Louise Downey and Usha Chakravarthy — reported that continuous therapy with ILUVIEN® (fluocinolone acetonide [FAc]) intravitreal implant leads to improved visual outcomes in chronic diabetic macular edema (DME) compared with intermittent therapies (laser, intravitreal steroid and anti-vascular endothelial growth factor [anti-VEGF]). In addition, earlier intervention with ILUVIEN — before visual acuity progressively worsens and as patients become less responsive to standard therapies — increased the likelihood of achieving a best corrected visual acuity (BCVA) of ≥20/40 (≥6/12).1
The authors conducted exploratory analyses of visual outcomes data from the Fluocinolone Acetonide in Diabetic Macular Edema (FAME) studies.1 FAME consisted of two randomized, double-blind, placebo-controlled, parallel group, multicenter studies; in which patients with chronic DME were treated with either 0.2 µg per day FAc (ILUVIEN; n=375), 0.5 µg per day FAc (n=393), or sham control (n=185) for 36 months. Any patient could receive rescue therapies after six weeks, at the discretion of the investigators.2
FAME revealed that patients with chronic DME had a greater response to ILUVIEN than those with non-chronic DME. More chronic DME patients treated with ILUVIEN achieved a ≥15-letter improvement in BCVA at month 36 (34.0%), compared with those with non-chronic DME (22.3%; Figure 1). Conversely, in the intermittent therapy arm; (ie, the sham control) a ≥15-letter improvement in BCVA was achieved by fewer patients with chronic DME (13.4%) than by those with non-chronic disease (27.8%).1
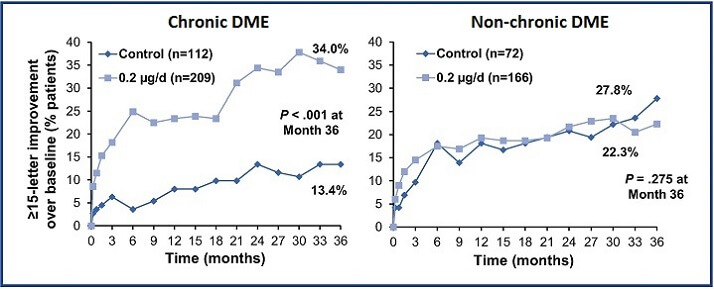
Figure 1: Proportion of patients with chronic and non-chronic DME achieving a ≥15-letter improvement in BCVA from baseline at month 36 following treatment with ILUVIEN or sham control.1
In the exploratory analysis by Downey et al., outcomes in FAME were examined according to baseline BCVA (≤20/64 [≤6/19], ≤20/80 [≤6/24], or ≤20/100 [≤6/30]) to assess the impact of pre-treatment visual acuity on post-treatment visual changes.1 As Dr Downey explained, “With this particular analysis…we tried to look at baseline visual acuity in a lot more detail because there is a growing realization that if you stratify your baseline vision you can get much more meaningful outcomes in terms of how well the drug is working”.3
Analysis of the FAME studies, presented in this same poster, investigated how DME chronicity and baseline vision influence the likelihood of achieving a BCVA of ≥20/40 at month 36 following treatment with intermittent therapy or ILUVIEN. According to Dr Downey, the benefits of helping more patients to reach a BCVA of ≥20/40 cannot be over-estimated, as this often equates to a real, functional benefit to the patient. “There is a growing realization that it is important not just to think about the percentage of patients who gain 15 letters or the mean visual acuity improvement, but to think about more of a functional measure of vision: 20/40 is often used because a patient whose vision has fallen to below [this level] would not meet the DVLA criteria for driving if that was their only eye. So, it is quite useful to think about how many of these patients — particularly if they are diabetic, they are often of working age, they need to maintain their drivers licence — would be able to do that.”3
Following treatment with either intermittent therapy or ILUVIEN, the percentage of chronic DME patients achieving a final BCVA of ≥20/40 at month 36 reduces as baseline vision falls (Figure 2). This highlights the importance of appropriate treatment as early as possible for optimal visual outcomes.1
Figure 2 also shows that significantly more patients achieved 20/40, in all BCVA groups, when treated with ILUVIEN compared to intermittent therapies. These data suggest, the continuous long-term release of FAc over 36 months provides a distinct benefit that cannot be achieved with intermittent therapies in patients with chronic DME.1
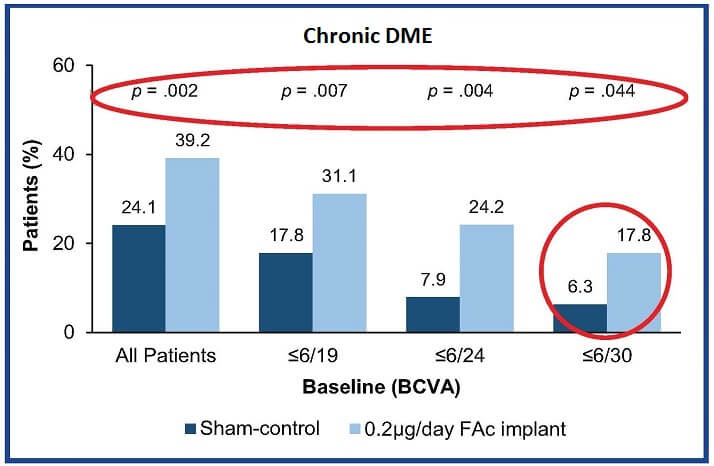
Figure 2: Patients with chronic DME achieving BCVA ≥20/40 at month 36, categorized by baseline BCVA. Adapted from Downey et al. 20151
Baseline BCVA had no impact on the chances of achieving visual acuity of ≥20/40 in non-chronic DME treated with either ILUVIEN or intermittent therapies (Figure 3).1
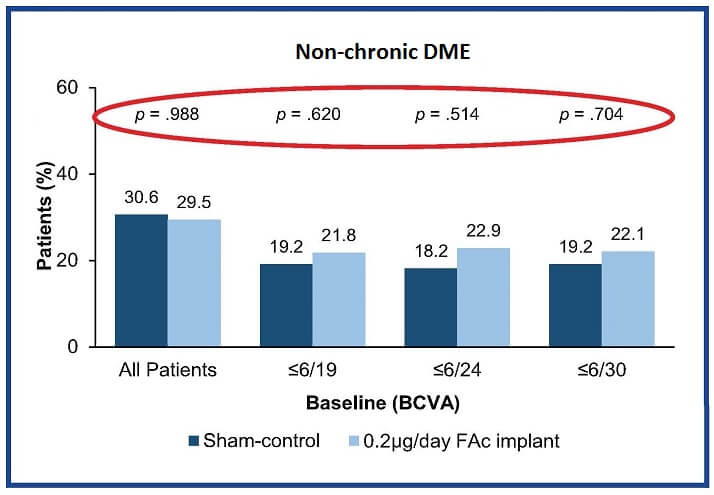
Figure 3: Patients with non-chronic DME achieving BCVA ≥20/40 at month 36, categorized by baseline BCVA. Adapted from Downey et al. 20151
These observations highlight the importance of appropriate treatment as early as possible in the natural history of chronic DME to optimize functional visual outcomes. “If you have chronic DME and you want to achieve a good visual acuity outcome, you would be better to access ILUVIEN earlier rather than later”, said Dr Downey.3
This leads to a key question “when does DME become chronic?” The FAME studies and the additional analyses presented here, define chronic DME as when a patient is insufficiently responding to anti-VEGF therapy.1,2 These patients have been experiencing progressive sight loss over several years and, in order to achieve the optimal outcome, it is essential to identify DME patients who are not responding sufficiently to standard therapies — such as anti-VEGFs — and to initiate ILUVIEN therapy as early as possible.

Louise Downey is a Consultant Ophthalmologist, specializing in medical retinal diseases, at Hull and East Yorkshire Eye Hospital, UK. She is actively involved in clinical trials relating to intravitreal therapies in retinal vascular diseases.

Please follow this link to access presentations delivered at the Alimera Sciences symposium http://www.events4healthcare.com/alimera_sciences/rco18052015.html
Look out for DME content developed by Alimera Sciences on this website throughout 2015. We hope it supports your knowledge of DME and ILUVIEN, and if you would like to contribute material for publication, please send your materials to dmecontenthub@hayward.co.uk, we’d be very pleased to consider your contributions.
REFERENCES 1. L Downey et al., “Exploratory analyses of long-term visual outcomes based on baseline vision in patients with chronic and non-chronic diabetic macular oedema (DMO) treated with fluocinolone acetonide (FAc)”. Poster presented at the Royal College of Ophthalmologists annual congress, Liverpool, UK, 19–21 May, 2015. Poster No. 221. 2. P Campochiaro et al., “Long-term Benefit of Sustained-Delivery Fluocinolone Acetonide Vitreous Inserts for Diabetic Macular Edema”. Ophthalmology. 2011; 118: 626-635. 3. Interview with Louise Downey. Hayward Medical Communications. 2015. 4. U Chakravarthy., “Long-term visual outcomes based on baseline vision in patients with chronic and non-chronic diabetic macular oedema (DMO) treated with fluocinolone acetonide (FAc)”. Presentation at the 14th EURETINA congress, London, UK; 11–14 September, 2014. 5. “Chronic DME patients with worst vision benefit most from continuous steroid therapy”. Cheryl Guttman Krader. Ophthalmology Times. 2015.
UK-ILV-MMM-0232 Date of preparation: June 2015
Founded in 2003, Alimera Sciences researches and develops innovative vision-improving treatments for chronic retinal disease. Alimera Sciences has developed and licensed ILUVIEN®, an intravitreal implant of 190 micrograms fluocinolone acetonide, for the treatment of vision impairment associated with chronic diabetic macular edema (DME), considered insufficiently responsive to available therapies.a ILUVIEN is the first DME treatment to deliver up to 36 months of continuous, low-dose corticosteroid by single injection.b In 2015, Alimera Sciences has partnered with The Ophthalmologist to facilitate the publication of independently created content on ILUVIEN and DME. Content will range from conference reports, case studies, and literature reviews to video interviews, presentations, and practical information surrounding the use and benefits of ILUVIEN. The word Alimera derives loosely from the Greek, to mean “day of truth”.c With a commitment to honesty, integrity, responsibility, candor, and trust, Alimera Sciences intend that this promotional information accurately and fairly represents the current state of knowledge of ILUVIEN and DME, and is useful to all healthcare professionals involved in DME and its treatment. a. ILUVIEN SPC. 2013 Available at: www.medicines.org.uk/emc/medicine/27636 (Accessed March 2015) b. Alimera Sciences. Available at www.alimerasciences.com (Accessed March 2015) c. Retina Today. 2011. Available at: http://retinatoday.com/pdfs/0111RT_Wall.Street.pdf (Accessed March 2015) UK-ILV-MMM-0167 Date of preparation: March 2015 www.alimerasciences.com enquiries@alimerasciences.com

