
Advertisement feature; Alimera Sciences’ recommendations for visualizing ILUVIEN® injection
Funded by Alimera Sciences. Contains promotional information. Please click here to view the prescribing information and adverse event reporting information.
A question was raised at the Alimera Sciences Ltd. (Alimera Sciences) symposium entitled “The importance of early recognition of chronic DMO; optimizing outcomes for the patient”, (Monday 18 May 2015; Liverpool, UK) at the Royal College of Ophthalmologists (RCOphth) Annual Conference [www.events4healthcare.com/alimera_sciences/rco18052015.html], regarding how the ILUVIEN® (fluocinolone acetonide) intravitreal implant (Alimera Sciences) can be visualized after injection. The following article summarizes what is stated in the summary of product characteristics (SPC), as well as the clinical insights proposed by some healthcare professionals on how to locate and visualize the implant within the vitreous humor.
Miss Tsaloumas, a consultant ophthalmic surgeon at Queen Elizabeth Hospital in Birmingham, UK, and Chair of the symposium, raised the point that,
“[…] 50% of the time I can’t see the ILUVIEN implant […] I look for it and I can’t see it.”
Considering that the ILUVIEN implant is rather small — at just 3.5 mm in length and 0.37 mm in width (Figure 1) — it is perhaps unsurprising that Miss Tsaloumas and many of her colleagues have asked for guidance on how to visualize the implant after application. To assist physicians, this article summarizes three techniques for implant visualization. These recommendations are based on the ILUVIEN SPC recommendations, observations and experiences from physicians acquired from scientific sessions at the Association for Research in Vision and Ophthalmology (ARVO) 2015 Annual Meeting, as well as from the previously mentioned Alimera Sciences symposium.

Figure 1. The ILUVIEN applicator (left) and the ILUVIEN implant compared to one cent euro coin (right).
First and foremost, physicians are advised to refer to the “Method of administration” section 4.2 of the ILUVIEN SPC (available at www.medicines.org.uk/emc/medicine/27636). According to the SPC, indirect ophthalmoscopy examination in the quadrant of insertion should be performed following intravitreal insertion of the ILUVIEN implant to ensure successful placement. Specifically, following intravitreal injection of ILUVIEN, the physician should “Remove the lid speculum and perform indirect ophthalmoscopy to verify the placement of the implant, adequate central retinal artery perfusion, and absence of any other complications.” The SPC goes on to highlight that scleral depression may enhance visualization of the implant.1
From a clinical perspective, Mr Fahd Quhill — a consultant ophthalmologist at the Royal Hallamshire Hospital in Sheffield, UK — described the technique he uses to identify the ILUVIEN implant during the Alimera Sciences symposium. To help visualize ILUVIEN, Mr Quhill recommends dilating the pupil before injecting ILUVIEN and, after the implant has been injected, he suggests waiting a short time before attempting to visualize it. To view the implant, Mr Quhill initially uses slit-lamp fundus biomicroscopy with a wide-field lens, examining the patient upright. If visualization still proves difficult, he then instructs his patients to lie flat on their back, so that the eye can be indented (scleral depression) and examined by indirect ophthalmoscopy, as recommended in the ILUVIEN SPC.1 Scleral depression changes the angle of the implant within the vitreous, thus, improving the contrast between ILUVIEN, and the surrounding tissue and fluid. Using this technique, Mr Quhill stated that, to date, he has never failed to locate an ILUVIEN implant in the eye.
Dr Alexander M Eaton — founder and director of the Retina Health Center, Fort Myers, Florida, USA — at the ARVO 2015 Annual Meeting described how he has successfully visualized the ILUVIEN implant using ultrasonography in B-scan mode to capture an image of the implant. In patients in whom the ILUVIEN implant cannot be easily visualized on a 20 diopter exam, B scan ultrasound can be helpful in identifying its location. ILUVIEN is picked up well on ultrasound images (as shown in Figure 2) and, thus, ultrasonography provides a useful tool for physicians.
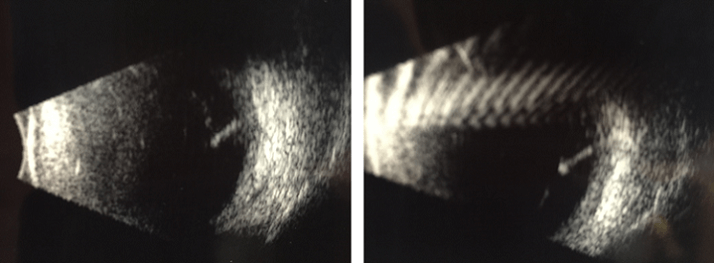
Figure 2. Ultrasound images (captured using the B-scan mode) of the ILUVIEN implant (located on the right-hand side of the images) within the vitreous. Images from Dr Alexander M Eaton presented at ARVO 2015.
The insights described in this article may help clinicians visualize the ILUVIEN implant within the vitreous. First, indirect ophthalmoscopy examination should be performed in the quadrant of insertion to ensure successful placement.1 If this does not enable visualization, recent experience shows that, clinicians can use dilation of the pupil to help improve the field of view or ultrasonography.
In addition, a follow-up appointment should be made between two and seven days after the implant has been first injected to check for possible complications. This visit provides the opportunity to locate the position of the implant within the vitreous and to monitor the patient for any potential complications.1
Please follow this link to access presentations delivered at the Alimera Sciences symposium www.events4healthcare.com/alimera_sciences/rco18052015.html
Look out for DME content developed by Alimera Sciences on this website throughout 2015. We hope it supports your knowledge of DME and ILUVIEN, and if you would like to contribute material for publication, please send your materials to dmecontenthub@hayward.co.uk, we’d be very pleased to consider your contributions.
REFERENCES
1. Alimera Sciences Ltd, “ILUVIEN Summary of Medical Product Characteristics”
2015. Available at: www.medicines.org.uk/emc/medicine/27636. (Accessed June 2015)
UK-ILV-MMM-0267
Date of preparation: June 2015
Founded in 2003, Alimera Sciences researches and develops innovative vision-improving treatments for chronic retinal disease. Alimera Sciences has developed and licensed ILUVIEN®, an intravitreal implant of 190 micrograms fluocinolone acetonide, for the treatment of vision impairment associated with chronic diabetic macular edema (DME), considered insufficiently responsive to available therapies.a ILUVIEN is the first DME treatment to deliver up to 36 months of continuous, low-dose corticosteroid by single injection.b In 2015, Alimera Sciences has partnered with The Ophthalmologist to facilitate the publication of independently created content on ILUVIEN and DME. Content will range from conference reports, case studies, and literature reviews to video interviews, presentations, and practical information surrounding the use and benefits of ILUVIEN. The word Alimera derives loosely from the Greek, to mean “day of truth”.c With a commitment to honesty, integrity, responsibility, candor, and trust, Alimera Sciences intend that this promotional information accurately and fairly represents the current state of knowledge of ILUVIEN and DME, and is useful to all healthcare professionals involved in DME and its treatment. a. ILUVIEN SPC. 2013 Available at: www.medicines.org.uk/emc/medicine/27636 (Accessed March 2015) b. Alimera Sciences. Available at www.alimerasciences.com (Accessed March 2015) c. Retina Today. 2011. Available at: http://retinatoday.com/pdfs/0111RT_Wall.Street.pdf (Accessed March 2015) UK-ILV-MMM-0167 Date of preparation: March 2015 www.alimerasciences.com enquiries@alimerasciences.com

