
Advertisement feature; Mr Frank Ahfat presents two patient cases where ILUVIEN is associated with rapid visual and anatomical responses in chronic diabetic macular edema responding insufficiently to other therapies
Funded by Alimera Sciences. Contains promotional information. Please click here to view the prescribing information and adverse event reporting information.
Mr Frank Ahfat — Consultant Ophthalmic Surgeon at the Maidstone and Tunbridge Wells NHS Trust in Kent, UK — was among the speakers at Alimera Sciences’ DME Grand Round Webinar in November 2014. Mr Ahfat’s presentation detailed some of his experiences in treating chronic diabetic macular edema (DME) with the long-acting corticosteroid, ILUVIEN® (fluocinolone acetonide [FAc]) intravitreal implant (Alimera Sciences).1
Mr Ahfat is experienced in ILUVIEN treatment; his presentation focused on two of the patients he has treated. The first patient was a male with type 2 diabetes mellitus and chronic DME. Despite multiple treatments with laser photocoagulation over a period of 10 years, the patient’s maculopathy continued to worsen. By 2012, his best corrected visual acuity (BCVA) had deteriorated to 6/12 in the right eye and 6/9 in the left eye. His central retinal thickness (CRT) had increased to 394 µm in the right eye.1
Treatment of the right eye with the intravitreal corticosteroid triamcinolone acetonide (TA; 4 mg) in May 2012 improved the patient’s BCVA to 6/6 and CRT decreased to 251 µm within one month (Figure 1). TA injection in the right eye was repeated in August 2012 but by March 2013, BCVA had again decreased to 6/24.1
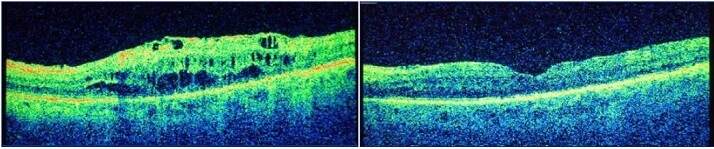
Figure 1. Optical coherence tomography scan before (left) and one month after (right) intravitreal injection of 4 mg triamcinolone acetonide.1
By July 2013, Mr Ahfat had received funding for use of the anti-vascular endothelial growth factor (anti-VEGF) drug ranibizumab (Lucentis®, Novartis Pharmaceuticals) and administered six injections of this into the patient’s right eye between July 2013 and March 2014. During this time, BCVA remained at 6/24 and CRT improved only slightly, from 414 µm to 392 µm (Figure 2).1
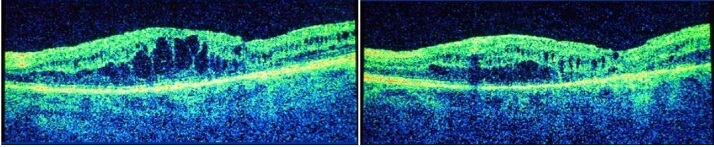
Figure 2. Optical coherence tomography scan before (left) and after (right) six intravitreal injections of the anti-VEGF ranibizumab.1
In June 2014, BCVA in the right eye had decreased further, to 6/36. Owing to his prior response to TA, the patient was treated with a single 0.2 µg ILUVIEN intravitreal implant. After just six weeks, BCVA improved to 6/24 and CRT decreased to 295 µm (Figure 3). After three months, BCVA had increased to 6/12 and CRT was reduced to 251 µm (Figure 3), compared with 392 µm prior to ILUVIEN treatment.1
Intraocular pressure (IOP) remained stable throughout follow-up after ILUVIEN injection. Prior to treatment, IOP was 16 mmHg in the right eye. Six weeks and four months after ILUVIEN treatment, IOP was 20 mmHg and 19 mmHg, respectively.1
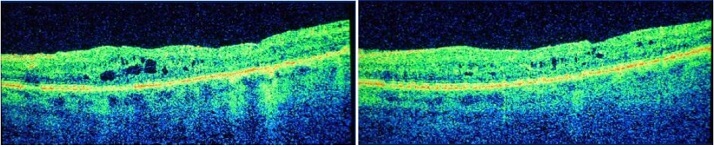
Figure 3. Optical coherence tomography scan six weeks after (left) and three months after (right) a single injection of ILUVIEN.1
The second patient described by Mr Ahfat was a female with type 2 diabetes mellitus, numerous co-morbidities and clinically significant chronic DME, diagnosed in 2008. Between December 2008 and December 2010 she received treatment in the right eye with three annual rounds of laser photocoagulation. Two injections of the anti-VEGF bevacizumab (Avastin®, Roche Products) were then administered in October and November 2011, with a further two injections in June and July 2013. Subsequently, five injections of ranibizumab were administered between November 2013 and May 2014. Despite this, treatment led to only a partial response. Mr Ahfat pointed out that the patient, who was wheelchair bound with a high disease burden, a history of dialysis, and leg ulcers found it difficult to adhere appropriately to the treatment visits required for anti-VEGF therapy, which impacted negatively on her quality of life. He therefore, treated her with a single injection of ILUVIEN in August 2014.1
Four weeks after ILUVIEN injection, BCVA improved from 6/24 to 6/18 and CRT from 355 µm to 270 µm. By two months, BCVA was 6/12 and CRT was 248 µm. A slight increase in IOP was seen from 19 mmHg before injection, to 20 mmHg at four weeks, and 21 mmHg at two months.1
These two case studies demonstrate that for chronic DME, insufficient long-term benefits were achieved from other therapies, whereas treatment with ILUVIEN produced visual and anatomical responses in these two patients within six and eight weeks, respectively, which were sustained with little effect on IOP. In addition, the requirement for only a single intravitreal injection of ILUVIEN can alleviate the burdensome multiple-treatment regimen associated with anti-VEGF therapy.
Mr Frank Ahfat is a consultant ophthalmic surgeon at the Maidstone and Tunbridge Wells NHS Trust. He is also the principal investigator in several national clinical trials on retinal diseases and a member of various professional bodies. Look out for DME content developed by Alimera Sciences on this website throughout 2015. We hope it supports your knowledge of DME and ILUVIEN, and if you would like to contribute material for publication, please send your materials to dmecontenthub@hayward.co.uk, we’d be very pleased to consider your contributions.

REFERENCE
1. F Ahfat, “Case studies in the treatment of diabetic macular oedema with a long-acting corticosteroid implant”, Presented at Alimera Sciences DME Grand Round Webinar, (2014) a virtual event sponsored by Alimera Sciences. Available at: events4healthcare.com/alimera_sciences/webinar25112014.html (Accessed May 2015).
UK-ILV-MMM-0230
Date of preparation: May 2015
Founded in 2003, Alimera Sciences researches and develops innovative vision-improving treatments for chronic retinal disease. Alimera Sciences has developed and licensed ILUVIEN®, an intravitreal implant of 190 micrograms fluocinolone acetonide, for the treatment of vision impairment associated with chronic diabetic macular edema (DME), considered insufficiently responsive to available therapies.a ILUVIEN is the first DME treatment to deliver up to 36 months of continuous, low-dose corticosteroid by single injection.b In 2015, Alimera Sciences has partnered with The Ophthalmologist to facilitate the publication of independently created content on ILUVIEN and DME. Content will range from conference reports, case studies, and literature reviews to video interviews, presentations, and practical information surrounding the use and benefits of ILUVIEN. The word Alimera derives loosely from the Greek, to mean “day of truth”.c With a commitment to honesty, integrity, responsibility, candor, and trust, Alimera Sciences intend that this promotional information accurately and fairly represents the current state of knowledge of ILUVIEN and DME, and is useful to all healthcare professionals involved in DME and its treatment. a. ILUVIEN SPC. 2013 Available at: www.medicines.org.uk/emc/medicine/27636 (Accessed March 2015) b. Alimera Sciences. Available at www.alimerasciences.com (Accessed March 2015) c. Retina Today. 2011. Available at: http://retinatoday.com/pdfs/0111RT_Wall.Street.pdf (Accessed March 2015) UK-ILV-MMM-0167 Date of preparation: March 2015 www.alimerasciences.com enquiries@alimerasciences.com

