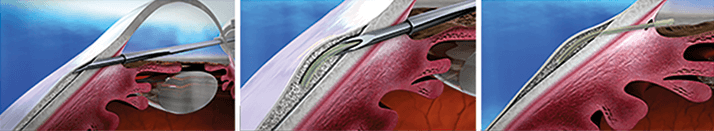
Hydrus Microstent (Ivantis Inc.) The world’s first “intracanalicular scaffold,” the Hydrus Microstent is approximately the size of an eyelash and is made from an elastic, biocompatible nickel-titanium alloy. When inserted, it bypasses the trabecular meshwork and dilates Schlemm’s canal to lower IOP. The implant is currently under evaluation in the Hydrus II and Hydrus IV clinical trials.
CyPass Micro-Stent (Transcend Medical) A supraciliary stent that creates a controlled outflow pathway to the suprachoroidal space. Openings all along the length of the stent tubing allow aqueous flow. The CyPass Micro-Stent has been clinically available in Europe since 2009, it is currently only available in the United States by participation in clinical trials. Xen Gel Stent (Aquesys, Inc.) Made of a soft, collagen-based gelatin, the Xen is compressible and conforms to the ocular tissue, minimizing issues associated with synthetic materials. It can be implanted either alone or in conjunction with cataract surgery. CE marked, the stent is classified as an investigational device in the United States and is therefore only available under an Investigational Device Exemption (IDE).
InnFocus Microshunt (InnFocus Inc.) Implanted through an ab externo needle track, the Microshunt moves aqueous fluid from the anterior chamber to a subconjunctival or sub-Tenon flap. It is made of a proprietary material called SIBS, previously used in cardiac stents and shown to cause minimal inflammation. The Microshunt received a CE mark in 2012 and is currently undergoing the FDA clinical trial approval process in the United States. iTrack Canaloplasty Microcatheter (Ellex) Canaloplasty aims to increase conventional outflow by catheterizing and dilating Schlemm’s canal. The iTrack microcatheter allows circumferential cannulation, and ophthalmic viscoelastic device injection, and it has an illuminated tip for transscleral visualization during insertion. The iTrack has received both FDA marketing approval and the CE mark.
