
Contains promotional information by Alimera Sciences. Please click here to view the prescribing information and adverse event reporting information for ILUVIEN® (fluocinolone acetonide [FAc]).
NICE reviews new treatment options in DME Over the last two years, NICE has issued guidance on four pharmacological treatments for diabetic macular edema (DME):5-8 Lucentis® (ranibizumab, Novartis Pharmaceuticals Ltd.), Eylea® (aflibercept, Bayer Healthcare Ltd.), ILUVIEN® (fluocinolone acetonide [FAc], Alimera Sciences Ltd.), and OZURDEX® (dexamethasone intravitreal implant, Allergan Ltd.) (Table 1).4-11

Table 1. Pharmacological treatment options for DME.4-11
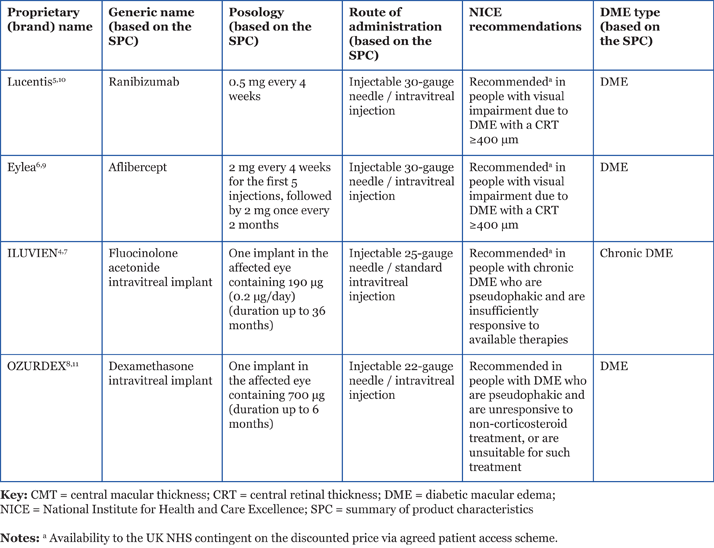
Vascular endothelial growth factor inhibitors NICE approved the use of ranibizumab for the treatment of DME in February 2013. The NICE appraisal concluded that ranibizumab was most cost-effective in people with visual impairment due to DME, with a central retinal thickness (CRT) of 400 µm or more.5 In July 2015, NICE also recommended aflibercept for use in people with DME if CRT is 400 µm or more.6 The NICE committee concluded that—based on both the evidence submitted and expert opinion—aflibercept was likely to have similar effectiveness to ranibizumab.6 The availability of both these anti-vascular endothelial growth factor (anti-VEGF) agents is contingent on their provision to the UK NHS at a discount agreed in a patient access scheme (PAS) with the Department of Health (DH).5,6 Both ranibizumab and aflibercept were appraised by NICE via the single technology appraisal (STA) process and, as they were reviewed separately, NICE did not make any recommendation on the clinical or cost-effectiveness of sequential treatment or switching between anti-VEGF options. Although some people with DME may respond better to either aflibercept or ranibizumab, it is not possible to predict this in advance.6 Physicians are, therefore, responsible for deciding the most appropriate treatment option on a case-by-case basis. Uptake of the guidance is likely to be influenced by clinical experience and comparative cost.
Corticosteroids—sustained delivery option for DME In November 2013, NICE approved the use of ILUVIEN in people with chronic DME who are both pseudophakic and insufficiently responsive to other treatments,7 such as laser and anti-VEGFs. The availability of ILUVIEN is contingent on its provision to the UK NHS at a discount agreed in a PAS with the DH.7 More recently, in July 2015, NICE also approved dexamethasone for use in people with both DME and a pseudophakic lens who have not benefited from other non-corticosteroid treatments or were unsuitable for such treatments.8 NICE assessed both ILUVIEN and dexamethasone via the STA process. Longer-acting steroid preparations (for example, ILUVIEN with a duration of up to 36 months, and dexamethasone intravitreal implant with a duration of up to 6 months)4,11 are of particular interest due to the reduced frequency of treatment required, which may provide a practical advantage compared with anti-VEGF agents. Studies have shown that in patients with chronic DME with frequent recurrences, longer treatment duration is beneficial.12 The release characteristics of corticosteroid intravitreal implants appear to mirror their efficacy: ILUVIEN offers a favorable pharmacokinetic profile (low dosage, low burst, sustained duration of release,13 and 36-month duration of efficacy), while dexamethasone may require re-treatment at 3- to 4-month intervals to maintain a dry macula and retain a good visual outcome.14 The risks of increased intraocular pressure and cataract formation need to be considered when using intravitreal steroid preparations; people who already have a pseudophakic lens are particularly suitable for treatment with these agents.4,11 Please refer back to Table 1 for further information on the pharmacological treatment options for DME.
Summary Given the progressive nature of DME, effective management is vital. NICE has considered the evidence and concluded that the use of the four treatments—ranibizumab, aflibercept, ILUVIEN and dexamethasone intravitreal implant—is only cost-effective in the proportion of the population specified in the relevant NICE guidance. Despite this, its positive recommendations mark a significant shift in the treatment paradigm for diabetic retinopathy, offering more treatment options compared with the previous standard of care, laser.

Dr. Farhat Butt is an ST4 trainee at Calderdale & Huddersfield NHS Foundation Trust, part of the Yorkshire and Humber Deanery. Dr. Rehna Khan is an ophthalmologist at Calderdale & Huddersfield NHS Foundation Trust. She graduated from Liverpool University Medical School and also holds a Certificate in Postgraduate Medical Education. She is the ophthalmology lead for undergraduate training and clinical lead for diabetic retinopathy, reflecting her special interests in diabetic retinopathy and age-related macular degeneration.
Look out for more DME content developed by Alimera Sciences on this website. We hope it supports your knowledge of DME and ILUVIEN. If you would like to contribute material for publication, please send your materials to dmecontenthub@hayward.co.uk, we’d be very pleased to consider your contributions.
REFERENCES 1. National Institute for Health and Care Excellence, “NICE Charter”, (2013). Available at: www.nice.org.uk/Media/Default/About/Who-we-are/NICE_Charter.pdf. Accessed July 16, 2015. 2. National Institute for Health and Care Excellence, “Guide to the process of technology appraisal”, (2014). Available at: http://www.nice.org.uk/article/pmg19. Accessed July 16, 2015. 3. National Institute for Health and Care Excellence, “NICE Statistics”, (2015). Available at: www.nice.org.uk/News/NICE-statistics. Accessed July 16, 2015. 4. Alimera Sciences Limited, “ILUVIEN 190 micrograms intravitreal implant in applicator. Summary of Medical Product Characteristics”, (2015). Available at: https://www.medicines.org.uk/emc/medicine/27224. Accessed July 16, 2015. 5. National Institute for Health and Care Excellence, “Ranibizumab for treating diabetic macular oedema (rapid review of technology appraisal guidance 237). NICE technology appraisal guidance 274”, (2013). Available at: www.nice.org.uk/guidance/ta274/. Accessed December 7, 2015. 6. National Institute for Health and Care Excellence, “Aflibercept for treating diabetic macular oedema. NICE technology appraisal guidance 346”, (2015). Available at: https://www.nice.org.uk/guidance/ta346. Accessed December 7, 2015. 7. National Institute for Health and Care Excellence, “Fluocinolone acetonide intravitreal implant for treating chronic diabetic macular oedema after an inadequate response to prior therapy (rapid review of technology appraisal guidance 271). NICE technology appraisal guidance 301”, (2013). Available at: www.nice.org.uk/guidance/ta301. Accessed December 7, 2015. 8. National Institute for Health and Care Excellence, “Dexamethasone intravitreal implant for treating diabetic macular oedema. NICE technology appraisal guidance 349”, (2015). Available at: https://www.nice.org.uk/guidance/ta349. Accessed December 7, 2015. 9. Bayer Healthcare Limited, “Eylea 40 mg/ml solution for injection (vial). Summary of Medical Product Characteristics 2014”, (2015). Available at: https://www.medicines.org.uk/emc/medicine/27224. Accessed July 16, 2015. 10. Novartis Pharmaceuticals Limited, “Lucentis 40 mg/ml solution for injection (vial). Summary of Medical Product Characteristics”, (2014). Available at: https://www.medicines.org.uk/emc/medicine/19409. Accessed July 16, 2015. 11. Allergan Limited, “Ozurdex 700 micrograms intravitreal implant in applicator. Summary of Medical Product Characteristics”, (2014). Available at: https://www.medicines.org.uk/emc/medicine/23422. Accessed July 16, 2015. 12. M Cabrera et al., “Sustained-Release Corticosteroid Options”, J Ophthalmol, 164692 (2014). PMID: 25140246. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4130028/. Accessed July 16, 2015. 13. Y Yang et al., “Intravitreal corticosteroids in diabetic macular edema”, Retina, 35, 2440–2449 (2015). Available at: http://journals.lww.com/retinajournal/pages/articleviewer.aspx?year=2015&issue=12000&article=00002&type=Fulltext. Accessed November 26, 2015. 14. WM Amoaku et al., “A review of therapies for diabetic macular oedema and rationale for combination therapy”, Eye, 1115–30 (2015). 15. F Butt et al., “Using EPR to identify eligible patients to implement NICE TA 301 (fluocinolone acetonide, ILUVIEN for diabetic macular oedema [DMO])”, presented at the Royal College of Ophthalmologists Annual Congress, 19–21 May, 2015, Leeds, UK (2015). 16. F Butt et al., “Identification of eligible patients to receive 0.2µg/day fluocinolone acetonide implant for patients with diabetic macular edema (DME)”, presented at the 15th EURETINA Congress, 17–20 September, 2015, Nice, France (2015). Date of preparation: November 2015 UK-ILV-MMM-0270
Founded in 2003, Alimera Sciences researches and develops innovative vision-improving treatments for chronic retinal diseases, such as diabetic macular edema (DME), dry age-related macular degeneration (AMD), and retinal vein occlusion. In 2015, Alimera Sciences partnered with The Ophthalmologist to facilitate the publication of independently created educational content surrounding DME, a serious retinal complication associated with diabetes, which is increasing in incidence with the increasing prevalence of diabetes worldwide. Published content will include articles ranging from basic science and disease processes to overviews of clinical data, different surgical procedures, comparisons of treatment options, and practical advice for managing diabetic patients. With a commitment to honesty, integrity, responsibility, candor, and trust, Alimera Sciences intend to provide educationally focused content to healthcare professionals across a wide range of topics in DME in order to both increase disease awareness and understanding, and to help improve patient outcomes. UK-ILV-MMM-0355 Date of preparation: August 2015 enquiries@alimerasciences.com

