
Contains promotional information by Alimera Sciences. Please click here to view the prescribing information and adverse event reporting information for ILUVIEN® (fluocinolone acetonide [FAc]).
The eyeball is a closed organ. As such, delivering efficient pharmacologic interventions can be challenging. Systemically administered medications face the problem of having to cross the blood–brain barrier and incur the possibility of systemic adverse events. Topically applied medications have to contend with a number of protective barriers in the eye (Figure 1), each of which has its own challenges; for example, drugs might bind to proteins in the tear film, and effective drug concentrations can be diluted as a result of tear turnover, which also accelerates drug clearance.1
Figure 1. Schematic representation of the eye and its protective barriers. Adapted from: Alila Medical Media/Shutterstock. Reproduced with permission.
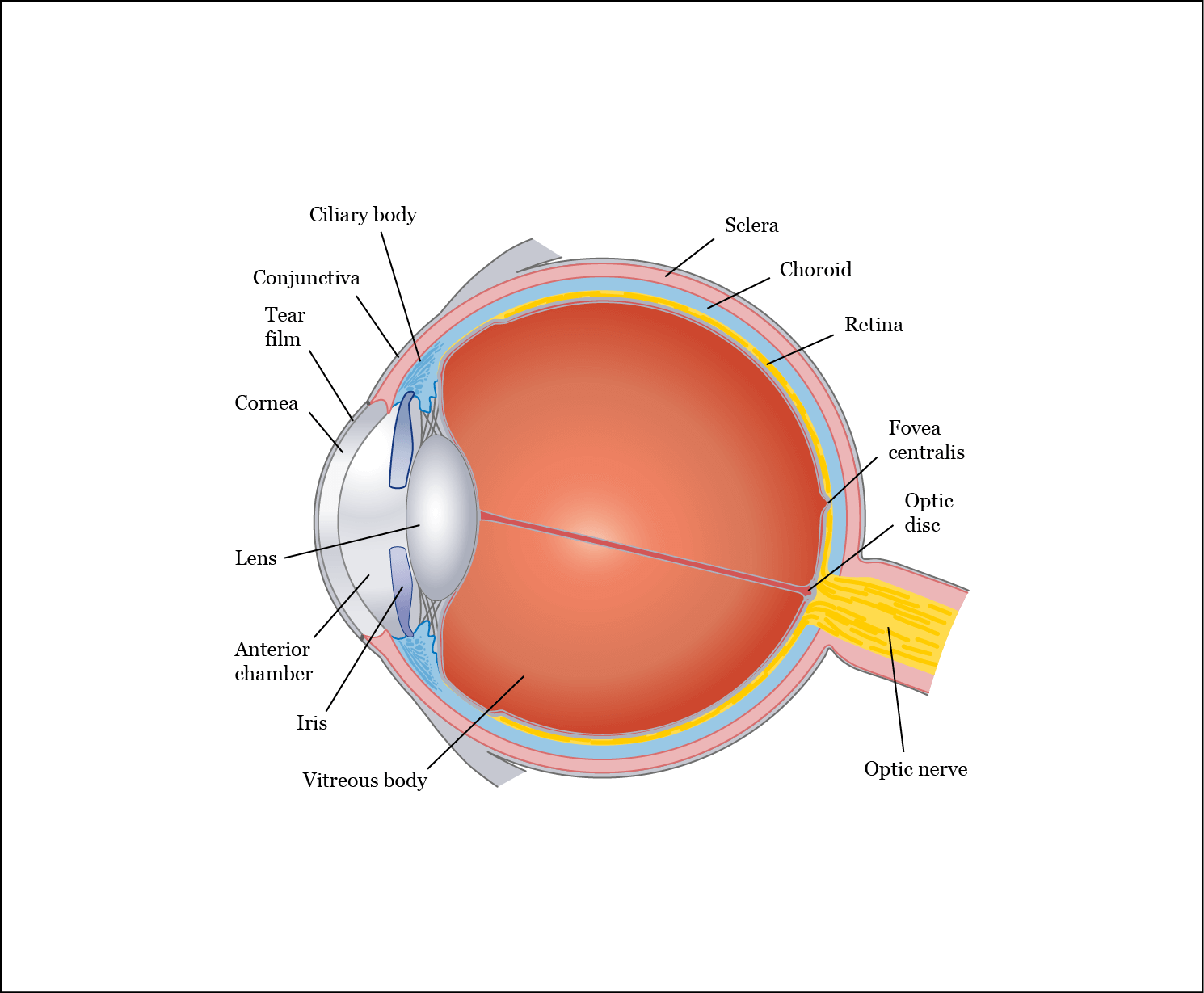
Drug delivery in the eye might be hindered by the cornea, a mechanical barrier made up of three layers, each of which possesses a different polarity and rate-limiting structure for drug permeation.1 Drugs can also face clearance from the conjunctiva (a thin, clear, moist membrane that coats the inner surfaces of the eyelids and the outer surface of the eye)2 through the blood and lymphatic systems.1 Furthermore, permeability through the sclera is strongly influenced by molecular radius, hydrophobicity and the retention of positively charged compounds within the sclera’s proteoglycan matrix.1 Given the aforementioned difficulties in eye permeability, it is estimated that only 1–3% of eye drop medication reaches intraocular tissues.1 One possible solution for bypassing eye barriers is direct intravitreal administration. However, treatments like anti-vascular endothelial growth factors (anti-VEGFs) need to be injected into the vitreal cavity, usually on a monthly basis and, thus, might place a considerable resource and cost burden on health services. In addition, patients report stress and anxiety due to repeatedly having to endure the unpleasant procedures. The requirement for regular clinic attendance can also be time consuming.3,4 Consequently, there is an increasing demand for therapeutic solutions to allow the protracted slow release of medication, reducing the number of injections required, and thereby decreasing the treatment burden for patients and health services.4 Because repeated intravitreal injections can occasionally result in retinal detachment and endophthalmitis, fewer injections could also better balance the therapeutic benefits against treatable side effects.1,3
Therapeutic options A number of therapeutic options are currently under development for a variety of ocular indications. These include implantable systems, injectable particulate systems, physical devices and intravitreal implants.
Implantable systems
- I-vation™ (SurModics, Inc.) intravitreal implant consists of a titanium helical coil and cap (length 0.5 mm, width 0.21 mm). The helix is encapsulated by triamcinolone-loaded non-biodegradable polymers (polymethylmethacrylate and ethylene vinyl acetate), with a predicted in vivo sustained delivery of a minimum of two years. The thin cap is designed to reside under the conjunctiva. Insertion of the implant requires conjunctival cut-down and placement through a 0.5-mm needle stick. This delivery system is non-biodegradable and remains in place after drug release is completed.1,5
- NT-501 ECT (Neurotech Pharmaceuticals, Inc.), designed as “encapsulated cell technology”, consists of a scaffold of six strands of polyethylene terephthalate yarn loaded with retinal pigment epithelium cells. This scaffold is surrounded by a semipermeable membrane capsule, which allows the outward diffusion of the recombinant ciliary neurotrophic factor and the inward diffusion of cell survival nutrients. The device is surgically implanted in the vitreous through a scleral incision, and is anchored by a single suture through a titanium loop.1
Injectable particulate systems (in development)
- Verisome™ (Icon Bioscience, Inc.) is a delivery platform for triamcinolone and consists of a translucent liquid, which forms a gel when mixed with saline. Drug secretion is expected to last up to one year from a single intravitreal injection.1
- Cortiject® (Santen Pharmaceuticals Co., Ltd.) contains the prodrug dexamethasone palmitate, encapsulated by a preservative-free emulsion composed of an oily carrier and phospholipid. Once released, the prodrug is de-esterified by a retina-specific esterase to produce dexamethasone (DEX). This system is designed to provide sustained release for 6–9 months.1
- RETAAC system comprises poly(lactic-co-glycolic acid) (PLGA) microspheres, loaded with triamcinolone. Its therapeutic benefits on diffuse diabetic macular edema (DME) are currently being evaluated.1
Physical devices
- The EyeGate II® Delivery System (Eyegate Pharmaceutical, Inc.) utilizes iontophoresis, a non-invasive technique by which topically applied drugs are driven through the sclera into the eye using a weak electric current.1 This technology might have the potential to replace both systemic administration and repeated intravitreal injections for posterior eye diseases.5
- Microelectromechanical systems (MEMS) are a powered reservoir system, in which the reservoir is implanted into the subconjunctival space and a flexible cannula inserted through incision into the eye. The reservoir can be refilled without additional surgical procedures.1
Intravitreal implants There are only two intravitreal implantable drug delivery systems currently available in the EU for the treatment of DME: ILUVIEN® (fluocinolone acetonide [FAc] intravitreal implant; Alimera Sciences, Inc.) and Ozurdex® (dexamethasone intravitreal implant; Allergan, Ltd.). ILUVIEN is licensed for the treatment of vision impairment associated with chronic DME that is considered insufficiently responsive to available therapies,6 such as laser and anti-VEGFs. Ozurdex is licensed for the treatment of adult patients with visual impairment due to DME who are pseudophakic or who are considered insufficiently responsive to, or unsuitable for, non-corticosteroid therapy.7 Although, at first glance, both of these systems look very similar, there are notable differences. Ozurdex is biodegradable and consists of a polymer PLGA matrix and 700 µg of DEX. It measures 0.46 mm in diameter and 6 mm in length. Administration is by intravitreal injection and retreatment can be performed after 3–6 months.7-9 The PLGA matrix slowly degrades to lactic and glycolic acid; thus, when the active agent is consumed, degradation products are water and carbon dioxide, leaving no residue in the eye.7 However, the mechanism of drug release in biodegradable implants can result in an initial drug-burst release, which can be a disadvantage when constant release kinetics are desired during therapeutic administration.10 This release profile was demonstrated in a study carried out in macaque monkeys, where levels of DEX dropped rapidly between Days 60 to 90, with levels slowly tapering after this period.8 ILUVIEN is a non-biodegradable rod-shaped cylindrical polyimide tube, 3.5 mm in length and 0.37 mm in diameter (Figure 2).6 ILUVIEN is small enough to be injected using an inserter with a 25-gauge needle, and is expected to provide a low dose of 0.2 µg/day FAc to the back of the eye for up to three years.6
Figure 2. ILUVIEN implant. Source: Data on file. Alimera Sciences.11 Reproduced with permission.
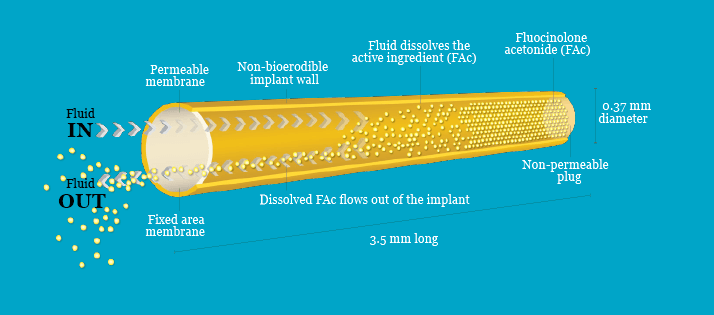
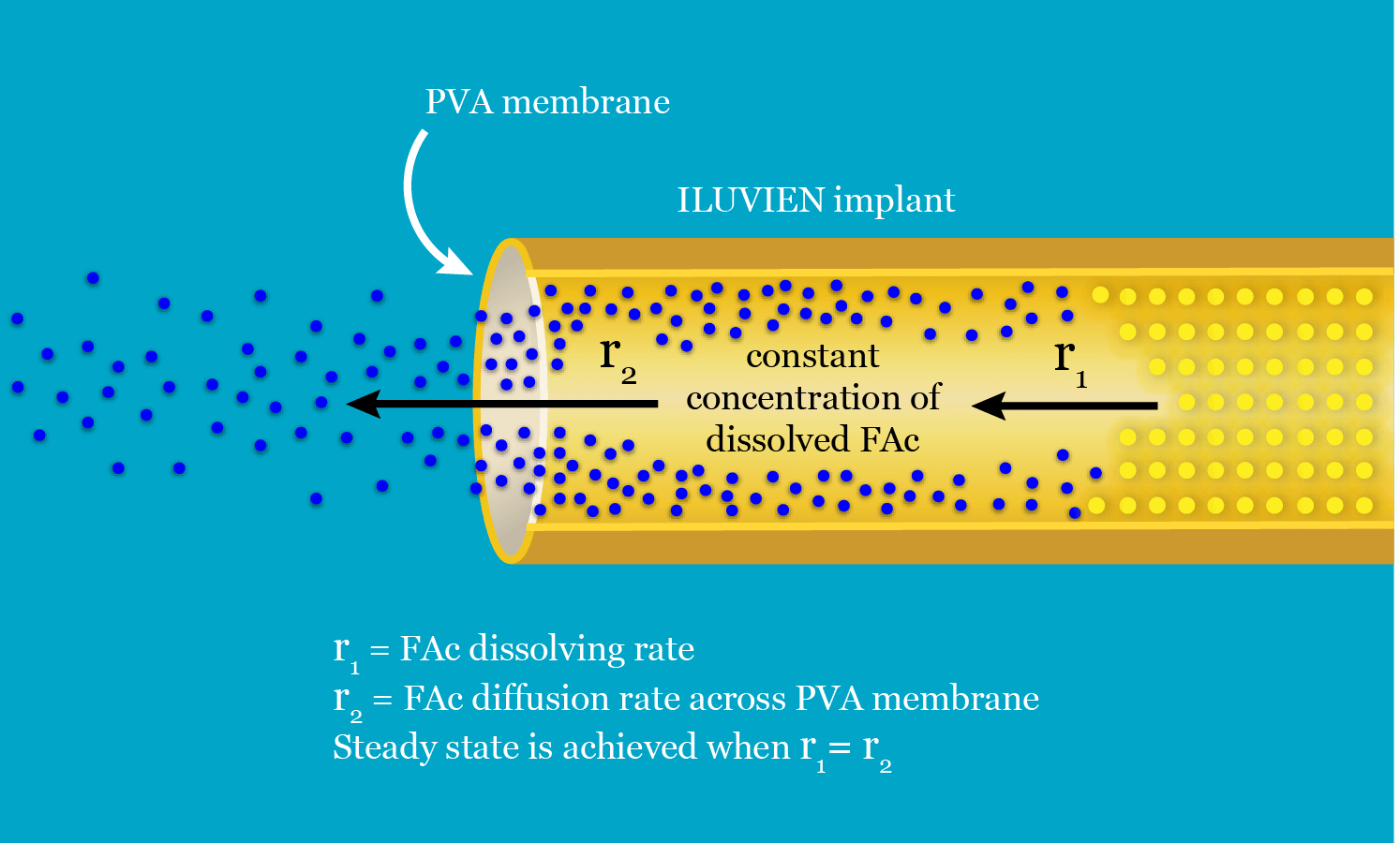
ILUVIEN is a reservoir-based system, in which one end of the cylinder is blocked by impermeable silicon. Fluocinolone acetonide is released through the polyvinyl acetate (PVA) polymer membrane at the other end. For FAc to be released, the drug must first be dissolved in water, creating a reservoir of dissolved drug within the implant; this process will proceed until the concentration of dissolved FAc in the reservoir reaches saturation.11 As a result, a chemical potential gradient of dissolved FAc is established across the PVA membrane between the reservoir and the vitreous, causing dissolved FAc to flow out of the implant through the membrane. This reduces the concentration of dissolved FAc in the reservoir, causing more FAc to dissolve. The rate of FAc release will be constant throughout the life of the implant if the concentration of dissolved FAc in the reservoir remains constant.11 A constant concentration of dissolved FAc in the reservoir is achieved when a steady state is reached; that is, when the rate at which the drug dissolves is equal to the rate at which the drug diffuses through the PVA membrane (Figure 3).11 This rate is determined by the diffusion coefficient of the drug across the PVA membrane and the size of the membrane.11
Figure 3. A steady state of fluocinolone acetonide (FAc) dissolving and FAc diffusing through the polyvinyl acetate (PVA) membrane enables steady, controlled release of FAc from the ILUVIEN implant. Adapted from: Data on file, Alimera Sciences.11 Reproduced with permission.
Pharmacokinetic studies of ILUVIEN have demonstrated that upon implantation, initial release of FAc was followed by a relatively steady release profile.12 Ozurdex and ILUVIEN are also available in the USA. Retisert® (Bausch and Lomb, Inc.) is the only other ocular implant approved by the US Food and Drug Administration. This is also a reservoir-based system for FAc administration, which requires a surgical procedure for implantation. It is not currently available in the EU.1
Future developments Ophthalmic drug manufacturers are continuing to develop novel delivery systems, such as ocular implants, which aim to provide longer drug availability, less frequent dosing, increased efficacy, and fewer adverse reactions. The use of innovative drug delivery systems is an essential strategy for ophthalmic drug manufacturers not only to support the delivery of new drugs but also to extend the commercial life of their current products.13 Novel therapeutic agents in late development stages pose an area of future growth for ophthalmic therapies. At present, research and development in ophthalmology is focused on slow-release anti-VEGFs, rho kinase inhibitors, integrin antagonists and anti-inflammatory agents, among other therapies.14
Look out for more DME content developed by Alimera Sciences on this website. We hope it supports your knowledge of DME and ILUVIEN, and if you would like to contribute material for publication, please send your materials to dmecontenthub@hayward.co.uk, we’d be very pleased to consider your contributions.
REFERENCES
- N Kuno, S Fuji, “Recent advances in ocular delivery systems”, Polymers, 3, 193–221 (2011). PMID: 23153114. doi:10.3390/polym301093.
- MedicineNet, “Definition of conjunctiva”, (2015). Available at: www.medicinenet.com/script/main/art.asp?articlekey=9893. Accessed September 8, 2015.
- Optician online, “Eye treatment of the future: drugs and other approaches”, (2014). Available at: www.opticianonline.net/eye-treatment-future-drugs-approaches/. Accessed September 8, 2015.
- S Oyetunde, S Sivaprasad. “The impact of injection therapy for ophthalmology patients with DME or RVO”, poster presented at the 6th World Congress on Controversies in Ophthalmology, 26–29 March, 2015, Sorrento, Italy, Poster No. 36 in Group A (2015).
- N Haghjou et al., “Sustained release intraocular drug delivery devices for treatment of uveitis”, Journal Of Ophthalmic and Vision Research, 6, 317–329 (2011). PMID: PMC3306122.
- Electronic Medicine Compendium, “Summary of product characteristics for ILUVIEN”, (2015). Available at: www.medicines.org.uk/emc/medicine/27636. Accessed November 27, 2015.
- Electronic Medicine Compendium, “Summary of product characteristics for Ozurdex”, (2015). Available at: www.medicines.org.uk/emc/medicine/23422. Accessed November 27, 2015.
- JE Chang-Lin et al., “Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant”, IOVS, 52, 80–86 (2011). PMID: 20702826.
- W Amoaku et al., “A review of therapies for diabetic macular oedema and rationale for combination therapy”, Eye (advance online publications), 1–16 (2015). PMID: 26113500.
- WW Yang, E Pierstorff, “Reservoir-based polymer drug delivery systems”, Journal of Laboratory Automation, 17, 50–58 (2012). PMID: 22357608.
- Data on file. Alimera Sciences.
- T Bertelmann, A Messerschmidt-Roth, “Intravitreal ILUVIEN® implant for diabetic macular oedema – early case experiences”, European Ophthalmic Review, 7, 122–126 (2013).
- pSivida, Products. Available at: www.psivida.com/products.html. Accessed November 27, 2015.
- Signals and Systems Telecom, “The ophthalmic drugs market: 2015 – 2030 – opportunities, challenges, strategies and forecasts”, 2015. Available from: www.snstelecom.com/ophthalmicdrugs. Accessed November 27, 2015.
Date of preparation: November 2015
Founded in 2003, Alimera Sciences researches and develops innovative vision-improving treatments for chronic retinal diseases, such as diabetic macular edema (DME), dry age-related macular degeneration (AMD), and retinal vein occlusion. In 2015, Alimera Sciences partnered with The Ophthalmologist to facilitate the publication of independently created educational content surrounding DME, a serious retinal complication associated with diabetes, which is increasing in incidence with the increasing prevalence of diabetes worldwide. Published content will include articles ranging from basic science and disease processes to overviews of clinical data, different surgical procedures, comparisons of treatment options, and practical advice for managing diabetic patients. With a commitment to honesty, integrity, responsibility, candor, and trust, Alimera Sciences intend to provide educationally focused content to healthcare professionals across a wide range of topics in DME in order to both increase disease awareness and understanding, and to help improve patient outcomes. UK-ILV-MMM-0355 Date of preparation: August 2015 enquiries@alimerasciences.com

