
Contains promotional information by Alimera Sciences. Please click here to view the prescribing information and adverse event reporting information for ILUVIEN® (fluocinolone acetonide [FAc])
Dr Anne-Marie Firan, Consultant Ophthalmologist at Rotherham and Barnsley Hospitals, UK, and Professor Florica Ignat, Consulting Professor at the University of Medicine and Pharmacy of Craiova and former Head of Ophthalmology at Craiova University Hospital, Romania, presented an ePoster at the recent European Society of Retina Specialists (EURETINA) congress, which took place from 17 to 20 September 2015 at the Nice Acropolis in Nice, France.1 The ePoster, entitled “Patient cases of functional and structural benefits of bilateral treatment with fluocinolone acetonide for diabetic macular edema (DME) insufficiently responsive to available therapies”,2 described the results of treating two insulin-dependent diabetic patients with chronic DME with ILUVIEN® (fluocinolone acetonide [FAc], 0.2 µg per day, licensed dose) intravitreal implant (Alimera Sciences). Both patients had become insufficiently responsive to available therapies. Unlike earlier studies, where an FAc implant has been administered to a single eye, these two patients each received FAc implants sequentially to both eyes (that is, four eyes in total). The authors presented the background history that led to the patients requiring such treatment, and the results from both patients up to two months after receiving the FAc implants.3 New data showing results from both patients up to 12 months after receiving FAc implants is now also available.4

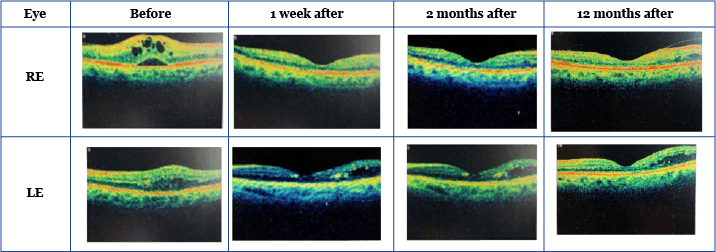
Patient 1: insufficiently responsive to laser and anti-vascular endothelial growth factor therapy (anti-VEGF) Patient 1, a 67-year-old woman with bilateral age-related macular degeneration and stage 3 chronic kidney disease, had been a type 2 diabetic for the past 15 years. Following assessment in mid-2013, she underwent bilateral argon laser pan-retinal photocoagulation for proliferative diabetic retinopathy and combined laser/anti-VEGF treatment for DME. Baseline visual acuity measured at this point was 6/60 in the right eye (RE) and 6/24 in the left eye (LE). However, despite a further seven anti-VEGF injections, there was no improvement in the LE visual acuity after 18 months of treatment, while the RE visual acuity had deteriorated to the extent that the patient could only count fingers at a distance of two meters.3 One week after receiving FAc implants, visual acuity in the RE improved. Previously only able to count fingers at two meters, Patient 1’s RE visual acuity improved to 6/38, and in the LE it improved from 6/24 to 6/15.3 After 12 months, visual acuity in the RE improved further to 6/9.5, while the LE remained relatively stable.4 Optical coherence tomography (OCT) showed a rapid reduction in central foveal thickness (CFT) after one week: CFT was reduced by 198 µm and 157 µm from the original baseline measurements in the RE and LE respectively (Table 1).3 These improvements continued, and after 12 months CFT was reduced by a further 113 µm and 104 µm in the RE and LE, respectively (Table 1).4
Table 1: VA, CFT and IOP pre- and post-0.2 μg/day FAc implant.3,4
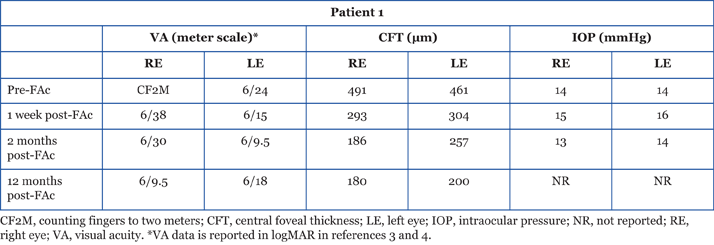
Improvements in retinal structure and integrity were also observed over this 12-month time frame (Figure 1).3,4
Figure 1: OCT images showing the retina before, and 1 week, 2 months, and 12 months after receiving bilateral 0.2 μg/day FAc implants.3,4 Reproduced with permission.
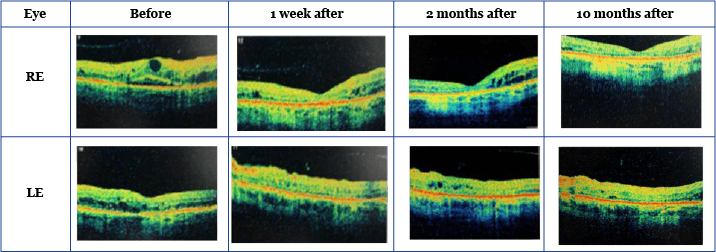
Patient 2: ineligible for anti-VEGF therapy and insufficiently responsive to laser and previous corticosteroid treatment Patient 2, a 68-year-old man, had a history of chronic obstructive pulmonary disease, hypertension, atrial fibrillation, and ischemic heart disease, including a myocardial infarction, for which he had received a coronary artery bypass. In addition, he had suffered from type 2 diabetes for the past 17 years and had developed diabetic peripheral neuropathy. The anti-VEGF therapy for his DME was contraindicated; therefore, he received intravitreal triamcinolone injections into both eyes, along with repeated macular grid argon laser treatment. At baseline, the patient was only able to count fingers at two meters with his RE and had a visual acuity of 6/19 in the LE. Although intravitreal triamcinolone injections improved visual acuity in the RE to 6/38, the LE deteriorated to 6/30.3 Similar to the results obtained with Patient 1, the visual acuity of Patient 2 also improved following insertion of the FAc implants – in this case, from 6/38 to 6/30 in the RE and from 6/30 to 6/18 in the LE, 10 months after receiving the implant.3,4 In addition, Patient 2 experienced a 191 µm and 152 µm drop in CFT over a period of 10 months in the RE and LE, respectively (Table 2).3,4
Table 2: VA, CFT and IOP pre- and post-0.2 μg/day FAc implant3,4
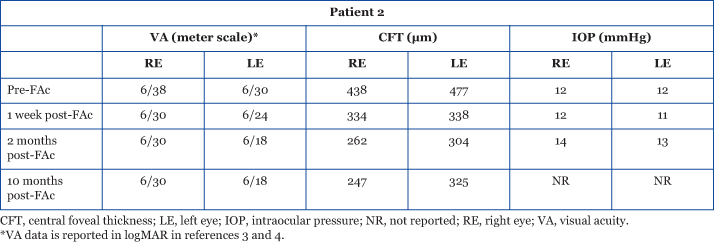
Improvements in retinal structure and integrity were also observed over this 10-month time frame (Figure 2).3,4
Figure 2: OCT images showing the retina before, and 1 week, 2 months, and 10 months after receiving bilateral 0.2 μg/day FAc implants.3,4 Reproduced with permission.
Neither of the patients developed increases in intraocular pressure (IOP) following insertion of the FAc implants, and both expressed satisfaction with the outcomes.3
Conclusion The authors concluded that bilateral therapy with ILUVIEN was effective in these two patients with bilateral DME, in terms of improvements in both visual acuity and retinal structural integrity, without incurring a concomitant increase in IOP.3 The results presented here demonstrate that both eyes can be treated with ILUVIEN. It must be cautioned, however, that bilateral treatment is performed under the proviso that the injections are conducted sequentially, on different days.3 This is in line with the current recommendations that an implant should not be administered to both eyes at the same visit as the patient's systemic and ocular response to the first implant should be known before treating the second eye.5
REFERENCES
- European Society of Retina Specialists. Available at: www.euretina.org/nice2015/default.asp. Accessed December 4, 2015.
- “15th ERETINA Congress Programme”, (2015). Available at: www.euretina.org/nice2015/PDF/EURETINA%20Nice2015%20Prog.pdf. Accessed December 7, 2015.
- AM Firan, F Ignat, “Patient cases showing functional and structural benefits of bilateral treatment with fluocinolone acetonide (ILUVIEN®; FAc) for diabetic macular edema insufficiently responsive to available therapies”, poster presented at the 15th EURETINA Congress, Nice, France, (2015).
- Additional data on file provided by Anne-Marie Firan.
- Alimera Sciences Limited, “ILUVIEN 190 micrograms intravitreal implant in applicator Summary of Product Characteristics’, (2015). Available at: www.medicines.org.uk/emc/medicine/27636. Accessed December 7, 2015.
Founded in 2003, Alimera Sciences researches and develops innovative vision-improving treatments for chronic retinal diseases, such as diabetic macular edema (DME), dry age-related macular degeneration (AMD), and retinal vein occlusion. In 2015, Alimera Sciences partnered with The Ophthalmologist to facilitate the publication of independently created educational content surrounding DME, a serious retinal complication associated with diabetes, which is increasing in incidence with the increasing prevalence of diabetes worldwide. Published content will include articles ranging from basic science and disease processes to overviews of clinical data, different surgical procedures, comparisons of treatment options, and practical advice for managing diabetic patients. With a commitment to honesty, integrity, responsibility, candor, and trust, Alimera Sciences intend to provide educationally focused content to healthcare professionals across a wide range of topics in DME in order to both increase disease awareness and understanding, and to help improve patient outcomes. UK-ILV-MMM-0355 Date of preparation: August 2015 enquiries@alimerasciences.com

