Stem cells are unspecialized cells that can, given the appropriate stimulation, give rise to differentiated cells of various kinds. Therapies based on stem cell treatments are considered to have tremendous potential in medicine. Ocular diseases are at the forefront of stem cell medicine, in part because the eye is a relatively simple, accessible and easily-monitored organ and also because stem cells in the eye are protected from potentially devastating immune attack by the organ’s immune privilege status.
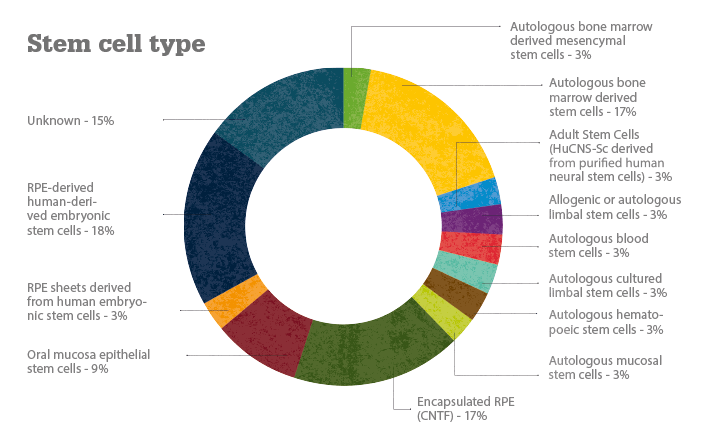
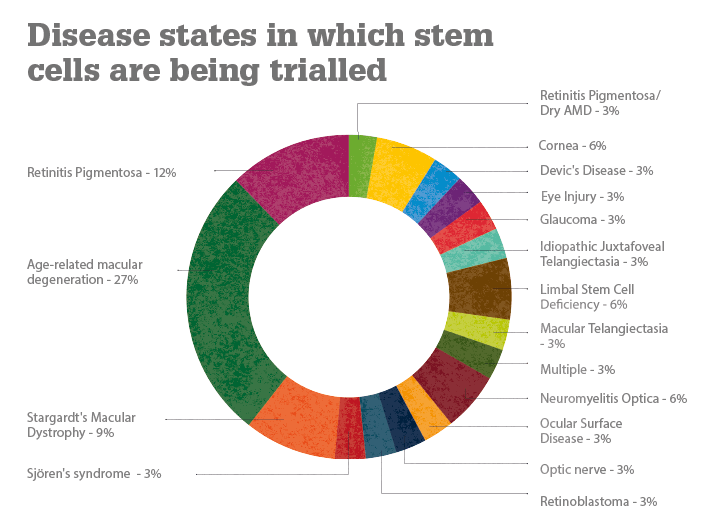
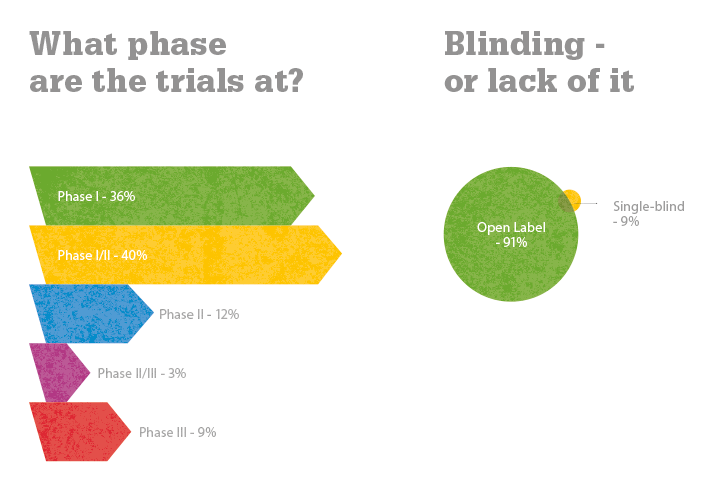
The various clinical trials covered here are being conducted in the United States, South America, Europe, Iran, India, South Korea, Taiwan, Japan and China. To date, more than 300 patients worldwide have received stem cell/cell therapy treatments. Positive results have been reported in both Stargardt’s disease and dry AMD trials (1). Transplanted human embryonic stem cell (hESC)-derived retinal pigment epithelial cells have resulted in engraftment and visual acuity gains (2); one dry AMD patient improved from 20/400 vision to 20/40 vision (3), which is good enough to obtain a driver’s license in most US states! Stephen Rose, Chief Research Officer at The Foundation Fighting Blindness, wrote, “Of course, it would be nice if all the parts of our bodies, including our retinas, came with extended warranties so you could just swap them out when they go bad. But now that I think about it, that’s what stem cells might do for us someday” (4).
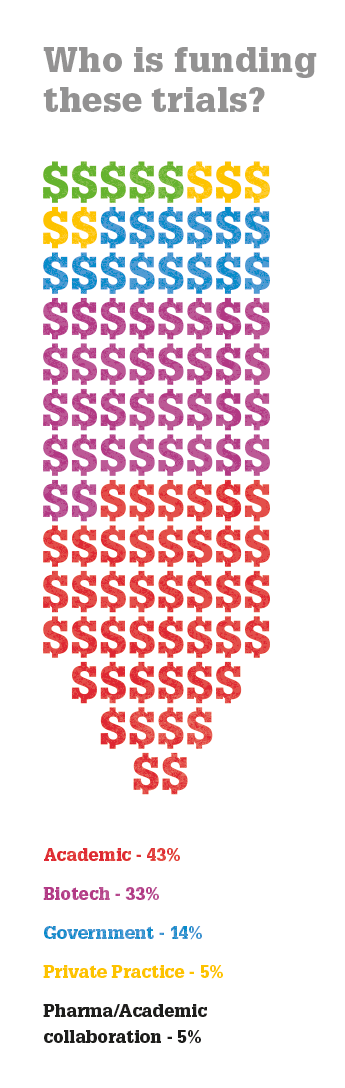
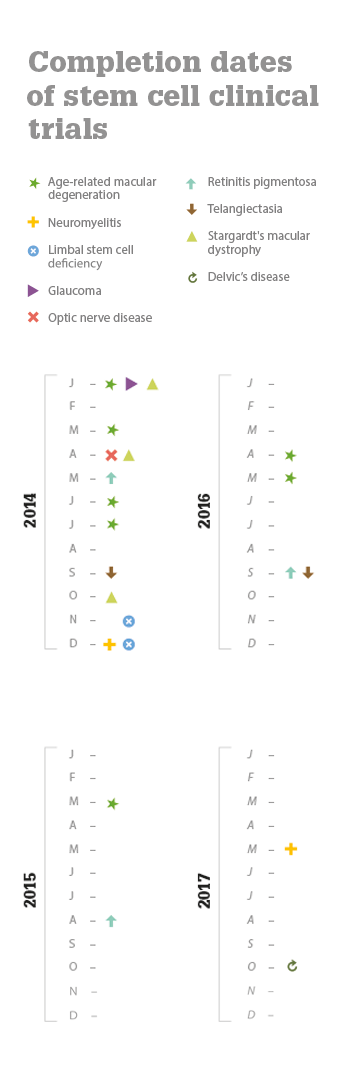
References
- SD Schwartz, J-P Hubschman, G Heilwell et al., “Embryonic Stem Cell Trials for Macular Degeneration: A Preliminary Report”, Lancet, 379(9817), 713–20 (2012). Advanced Cell Technology Achieves Clinical Milestone, Press Release, January 8, 2013. ACT Confirms Clinical Trial Participant Showed Improvement in Vision from 20/400 to 20/40 Following Treatment, Press Release, May 16, 2013. S Rose, “There’s More than One Way to Correct a Genetic Defect”, Eye on the Cure, http://www.blindness.org, April 11, 2012.
