- Fifteen years ago, as a student, Matteo Leonardi discovered his passion
- With partners, he has developed a company to build and market his product
- The device offers 24-hour IOP monitoring
- Development of a knowledge base is central to the company’s goals
- Other applications of the technology are in the pipeline
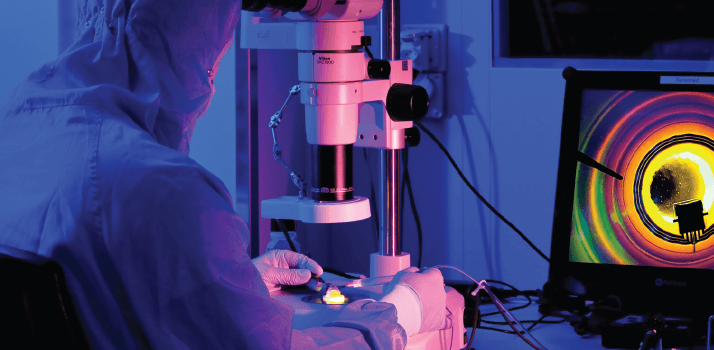
Here’s how to get long-term measurements on intraocular pressure (IOP). You place a sensor-containing contact lens on the eyeball of your patient and switch on the wireless recording device; the portable recorder tells you that everything is working well. The patient then leaves your clinic, goes about their everyday business as normal, and returns with a treasure-trove of IOP-related data 24 hours later. You Bluetooth it over to your laptop, and within seconds, you’re looking at the patient’s profile of spontaneous dimensional changes of the eye over the last day and night. You are then in the position to form an objective, informed diagnosis and a treatment plan for your patient.
Idea to Product…
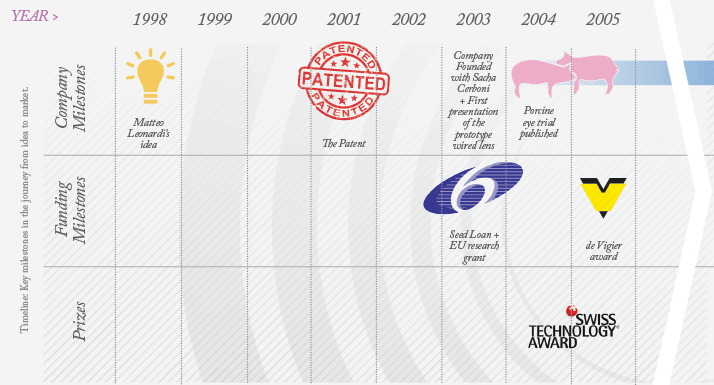
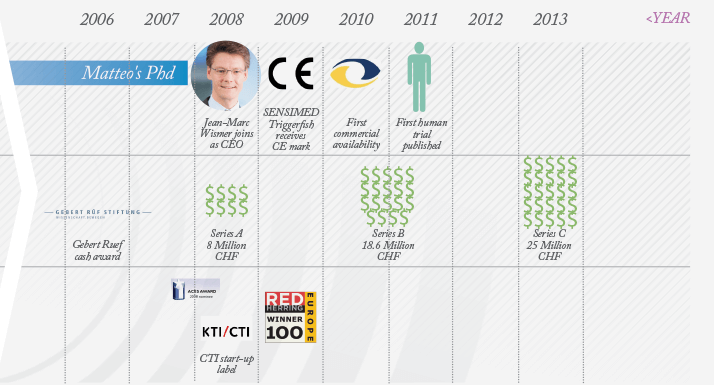
Matteo Leonardi, founder and Chief Technology Officer of Sensimed, decided to tackle long-term IOP monitoring back in 1998 while studying Biomedical Engineering at the Swiss Federal Institute of Technology (EPFL) in Lausanne. When the subject was discussed by a multidisciplinary group of engineers and physicians, a big idea emerged: a contact lens that could continually monitor IOP over a 24-hour period. Fifteen years later, the lens, Triggerfish, is on the market. Leonardi’s gentle demeanor belies the grit, determination and sheer perseverance he – and the team who have worked alongside him – have had to show to get this far. “Financing for innovation is very tight,” he explains, “and it has been a long journey.” Initially, financing wasn’t the biggest problem; it was time. Matteo was a research assistant at EPFL, pursuing the IOP project in his spare time. At that he point he was trying to determine if it could even be achieved using existing technology. By 2004 he was working on it full-time – it had become his PhD research topic – and he made a prototype that was used in preliminary clinical trials. By then, a patent had been granted to the EPFL for the device, which was licensed exclusively to a new company, Sensimed. Leonardi co-founded Sensimed with Sacha Cerboni, who brought expertise in health economics, quality assurance and regulatory knowledge – plus a fresh perspective – to the party. Today, Cerboni is responsible for Sensimed’s quality assurance and regulatory program. As momentum gathered, so did the requirement for cash. The Sensimed team managed to tap almost every possible source of Swiss start-up funding, and secured a coup by achieving funding for Triggerfish from the European Union (EU) Healthy Aims and EU Sixth Framework Programmes. But a different league of financing was required if the product was ever to be commercialized. This is where Jean-Marc Wismer came in. A veteran of the Swiss biotech scene, with extensive experience of both management and obtaining start-up funding, Wismer made an immediate impact. By the end of January 2008, Sensimed had closed Series A funding of CHF 8 million. This funding was crucial for Sensimed’s continued existence. Although the prototype had worked well and had provided excellent proof-of-concept, it had no possibility of meeting regulatory requirements. “We had to totally redesign the electronics, the telecommunications and the manufacturing procedures in order to satisfy medical and radio frequency legislation,” Leonardi explains. The costs were considerable: Wismer notes that Sensimed “burned through most of the CHF 8 million getting to the production models – on the industrialization, the clinical trials, and the marketing aspects.”…to Market
The next challenge was educating potential customers. “Our first surprisewas that while various advisors and the opinion leaders we consulted with were telling us that the device was great, ophthalmologists are more conservative than we thought, and it was difficult to move them from their usual practice and reference instruments,” Leonardi recalls. One barrier was that Triggerfish doesn’t measure IOP in the conventional units, mmHg. Rather, it senses a direct consequence of changes in IOP – ocular volume – via an embedded circular strain gauge in the lens. It reports circumferential changes at the corneoscleral area in millivolts and this is converted into arbitrary units. “When they first got this information, ophthalmologists didn’t know what to do with the data,” Wismer says. “There is an analogy with the electrocardiogram. The first doctors to use it looked at these traces and thought: what do I do with this? Now all doctors know how to decipher QRST peaks and work out what’s wrong.” Sensimed needed more time to develop and educate the market… and more funding. The company obtained Series B funding of CHF 18.6 million in 2010, and with it, some breathing space. The money financed early commercial activities and meant that Sensimed could transform into a full-blown medical device corporation. They spent time identifying prominent ophthalmologists who were likely to not only try Triggerfish, but to also adopt the device, validate the earlier clinical research and develop larger clinical trials. “Some ophthalmologists just get it straight away,” Leonardi says, “They realize that it gives you lots of info, that it gives you dynamic behavior information – such as how IOP drops when the patient wakes up.” Others need more time, and education, to understand the utility of our device. “Currently, ophthalmologists look at the optic nerve, try a treatment, give it six months, reassess, and repeat; it’s trial and error,” he states. “With Triggerfish, we show how the eye adapts to physiological stress, and we’re seeing different profiles in different disease states – indeed, we can identify the disease from the profile.” Can glaucoma really be reclassified according to the profiles and is IOP valid for diagnosis? “I don’t think we’re quite there yet,” is Leonardi’s answer. “We are just about to differentiate between stable and progressing patients, and to predict at-risk patients who will progress to glaucoma.” In terms of educating the market, he states that Sensimed have “good visibility; we’ve put out papers, abstracts, white papers, and presented at the big congresses.”How To Build Your Own Company
Do you have a great idea for a drug, device or service that will revolutionize ophthalmology? Here are tips from Matteo Leonardi and Jean-Marc Wismer on how to get it off the ground. “Perseverance. We had so many hard times; so many reasons to quit. To succeed, it almost feels like you have to have perseverance and resilience in your DNA.” “Think carefully about your business model. The finance situation has dramatically changed in the last decade; there’s less money to go around. So think really carefully before embarking on an expensive business model.” “Educating your market costs more than you think. It consumes money, so do not underestimate that when doing your calculations.” “Murphy was an optimist. You need a plan B and C and D. More sweat, more time and more money.” “Passion is a major asset. If you like the domain you are into, have a vision for a great contribution to society, it will feed you with the extra energy you need to carry you through hard times.” “In a start-up environment the team is crucial, as there are few to face the big challenges; you need everybody to think big and be willing to work in cross-functional projects.”