
- Efficient topical drug delivery is extremely difficult, thanks principally to the tear layer and the anatomy of the ocular surface
- Topical drug delivery to the posterior segment is, today, totally unachievable, hence the need for intravitreal injections
- Nanotechnology – both nano-scale formulations and smart coatings – has the potential to solve both problems
- This is likely to be game-changing – the reduction of intravitreal injections for wet AMD represents just a tiny fraction of nanotech’s potential
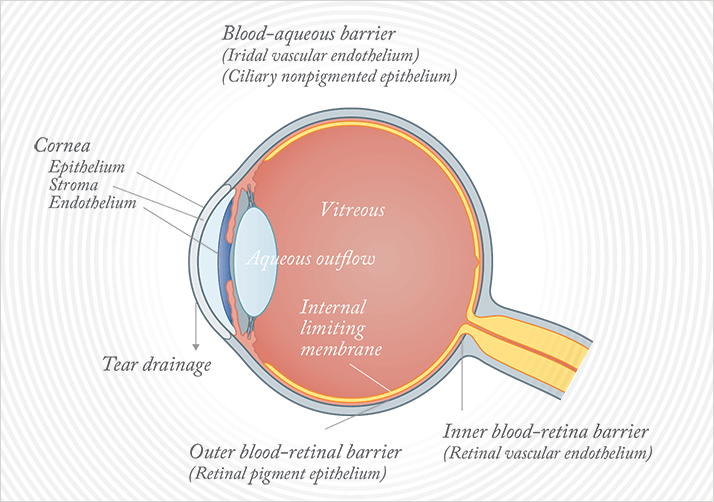
Ophthalmology has a problem. The problem is ocular drug delivery, and it’s as old as the first drugs used on the eye. In fact, the problem is the eye; its anatomy, and its protective mechanisms. Even if you want to apply a topical therapeutic to the cornea, you have big issues (Figure 1). The aqueous layer of the tear film not only rapidly washes away anything in an aqueous formulation, but the mucus layer gets in the way too: it contains mucins. These highly glycosylated, sticky molecules are wonderful for arresting the progress of foreign objects and pathogens towards the cornea, binding them, and preparing them for removal. But the tear film does the same with topically applied drugs. If you’re thinking of approaching the problem with a systemically administered drug, most won’t get there: the blood-ocular barrier will prevent most drugs from passing efficiently into the eye. For ocular surface disease, the mainstay option is topical therapy, despite the inefficiencies associated with its use.
If you want to deliver a drug – say ranibizumab or aflibercept – to the back of the eye, your only option is intravitreal injection. These are big drugs – 48 and 97 kDa, respectively – and topical application is totally ineffective in delivering them to the retina. These anti-VEGF agents also have powerful systemic side effects, but this is where the restricted passage of large molecules across the blood-ocular barrier helps; once drugs are injected into the eye, they tend to remain there for extended periods, minimizing systemic effects. But the fact that these big, anti-VEGF agents are the only effective drugs currently available for wet age-related macular degeneration (AMD), and absolutely have to be delivered by intravitreal injection, means that ophthalmology clinics are overflowing with aging baby-boomers, waiting for their monthly Lucentis or bimonthly Eylea injection. This is far from ideal – but it is the status quo. As the population ages, and ever-increasing numbers develop wet AMD, on this aspect alone, things have to change.
Why small matters
A nanometer is really small: one billionth of a meter. Why, as physicians, should you care? Well, across medicine, nanotechnology is allowing many major potential advances: improved vaccines, speedier and more sensitive diagnostic tests, and most importantly for ophthalmology, dramatically improved – and targeted – drug delivery. So how can nanotechnology specifically help us to overcome the challenges described? Well, nano-scale drug particles are more easily and rapidly absorbed by tissue than their bigger-scale counterparts, and their use tends to be associated with a reduced dosage requirement to achieve the same efficacy – which should result in fewer side effects as a side benefit. We can also modify them, make them hit the desired target by coating them with other molecules – indeed, we can engineer the surface properties of nanoparticles to target them for delivery to specific locations. For example, if you modify the surface to have a ligand that binds a certain cell type, or one that undergoes receptor-mediated endocytosis on a target cell, then you can have a highly specific targeting mechanism for drug – or gene – delivery. Nanotechnology helps break through the eye’s barriers to drug delivery too (Figure 2). Modified nanoparticles can penetrate the mucus barrier (Figure 3), with lower doses and a longer duration of action – resulting in better treatments for ocular surface diseases. If you can penetrate the cornea, you can reach the anterior chamber, which could lead to improved treatments for glaucoma and development of new treatments for anterior chamber diseases. There may even be the possibility of developing nanoparticle eye drops that can take therapies through to the back of the eye. Imagine, an effective treatment that doesn’t require injection. How likely then is it that you could one day be treating your patients with nanotechnology therapies? Let’s look at the evidence.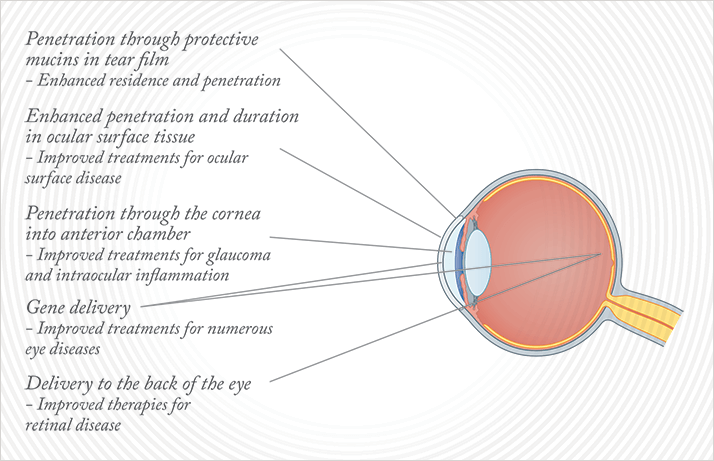
Smaller steroids
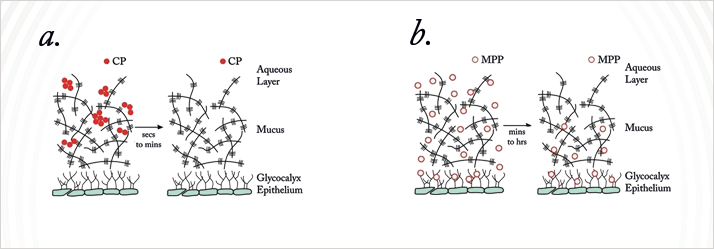
Many of you probably know the topically administered corticosteroid, loteprednol etabonate (LE), or Lotemax. You may have used it to treat post-surgical ocular surface inflammation, or other steroid-responsive inflammatory diseases of the cornea or conjunctiva. It has other benefits, including that its use is associated with a lower risk of intraocular pressure (IOP) elevation than other steroids. It does, however, have some of the same drawbacks that other eye drops currently have: it gets stuck in the mucus layer of the tear film, the drug particles aggregate, they’re distributed poorly and eliminated quickly. So in its current form, as with almost all eye drops, you have to overdose to account for these losses. Nanosuspensions do better, but the mucus barrier is tough to bypass (1).One of Robert Langer’s former students, Justin Hanes, has led the development of polyethylene glycol (PEG)-coated nanoparticles that penetrate the mucus layer of the tear film at a far faster rate than unmodified, ‘vanilla’ nanoparticles.
Conventional nanoparticles do not penetrate the mucus layer of the tear film.
PEG-coated nanoparticles rapidly penetrate the tear film.
The coated nanoparticle can rapidly make its way through the mucus; the plain nanoparticle remains stuck (2). Presumably this, plus LE, should result in better penetration of the mucus and therefore drug delivery (2), right? Another of Robert’s former students, Hongming Chen, is involved in precisely that, leading a team that is combining the mucus-penetrating particles (MPPs) with LE to create a better-penetrating – and hopefully safer and more effective – formulation. Can this be done? Results from a rabbit model look good. Hongming and her team compared the ocular pharmacokinetics of nano-scale 0.4% LE mucus penetrating particles (LE-MPP) formulation with the longest-acting currently available ophthalmic formulation of LE, 0.5% Lotemax gel (3). The results looked good, and main findings were clear; despite the LE-MPP dose being a fifth lower, it provided equal or better drug delivery than the gel formulation, reaching greater maximum concentrations at a faster rate, and taking longer to be cleared from the eye, leading to greater drug exposure (Figure 4a).
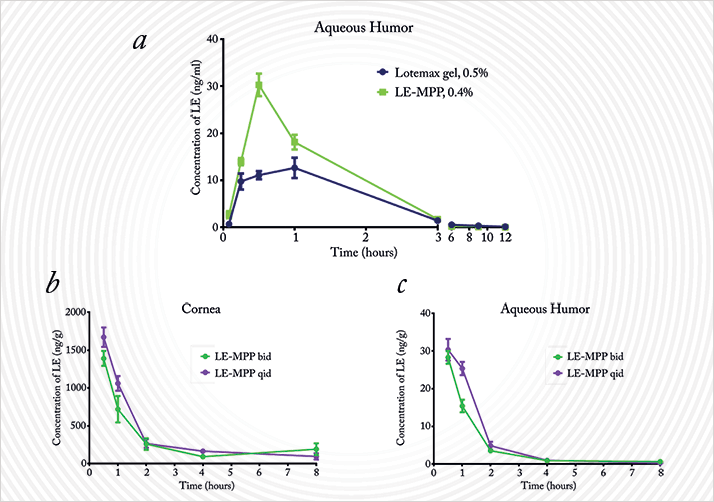
These pharmacokinetic characteristics led Hongming and her team to investigate whether the LE-MPP formulation needs to employ the four-times-a-day administration that LE eye drops currently require. Naturally, if they could get it lower, all the better for patient compliance (4). But could twice-a-day (bid) dosing be as effective as a four-times-a-day (qid) regimen? Again, in rabbits, the team compared LE concentrations in the cornea and the aqueous humor after twice- and four-times-a-day dosing (Figure 4b,c). Both protocols resulted in similar maximum drug concentrations over a similar timecourse. Of note, the total drug exposure was slightly reduced (~15–20 percent) when administered bid rather than qid – but remember, this was with a 50 percent lower cumulative dose. So we have increased penetration and a longer duration of action in ocular tissues, by making them at the nano scale and coating them with PEG – a material that the FDA consider to be GRAS – generally regarded as safe. The next step is clinical evaluation, and the first patient was dosed in a phase III trial of LE-MPPs back in June.
Solving the insoluble and making things gel
Here’s an example from another research group, who produced a calcium phosphate nanoparticle formulation of the carbonic anhydrase inhibitor, methazolamide (5). As this IOP-lowering drug is water-insoluble, it won’t dissolve in the aqueous tear film layer. This means that it’s rapidly cleared from the ocular surface, and unsurprisingly, results in poor ocular bioavailability. Calcium phosphates are both biocompatible and non-toxic (they’re important constituents of bone and constantly circulate in the bloodstream) and are readily taken up by cells. Helpfully, they are easily transported through even the smallest of capillaries, and this property enables them to accumulate in target cells. You can also adsorb methazolamide to calcium phosphate nanoparticles, and the combination of the two gives you a much better therapy than methazolamide alone. Rabbit studies (5) have demonstrated a longer duration of action, and a tremendous IOP-reduction response with methazolamide nanoparticles compared with the current formulation of the drug (Figure 5). Even insoluble issues like insolubility can be solved with nanoparticles, it seems.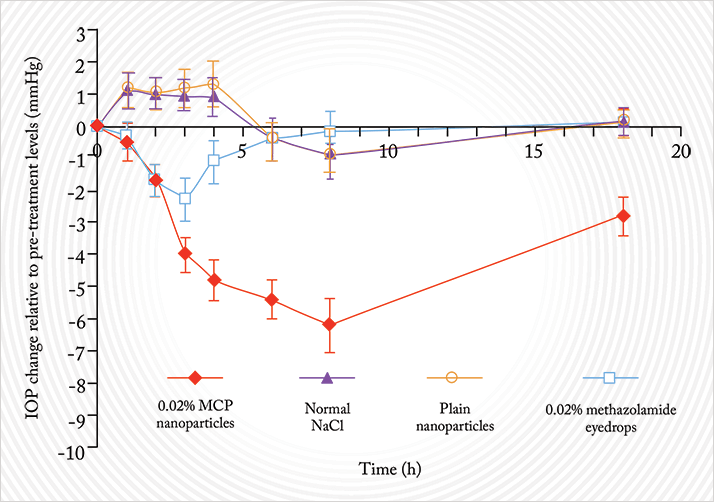
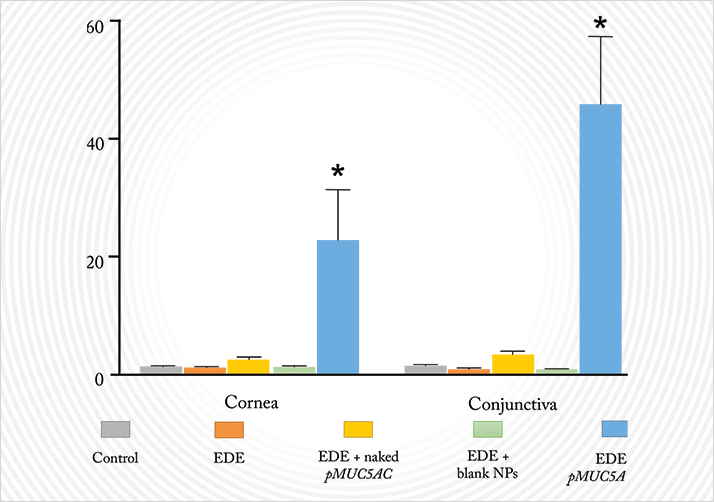
Interestingly, nanoparticles made from gelatin also hold promise according to a group in Valladolid, Spain, who have been working on a potential gene-based treatment for dry eye disease (6). They are trying to transfect a DNA plasmid that contains a copy of the MUC5AC gene – a gene that encodes for an important mucin that plays a central role in tear homeostasis. MUC5AC is downregulated in many ocular diseases with a dry-eye phenotype, like keratoconjunctivitis sicca and Sjögren’s syndrome. In mice with scopolamine-induced experimental dry eye (EDE), the group showed the application of naked pMUC5AC did not significantly increase MUC5AC expression relative to control mice (without EDE), EDE mice or EDE mice that had received gelatin nanoparticles alone. The combination of the plasmid and the nanoparticles, however, did result in a big increase in MUC5AC in both the cornea and conjunctiva (Figure 6). You can ascertain the extent of dry eye symptoms with fluorescein staining and tear production assays: both were significantly improved with pMUC5AC-nanoparticles, indicating that they were alleviating the symptoms of dry eye – no other intervention or control improved either parameter.
Persistence at the posterior segment
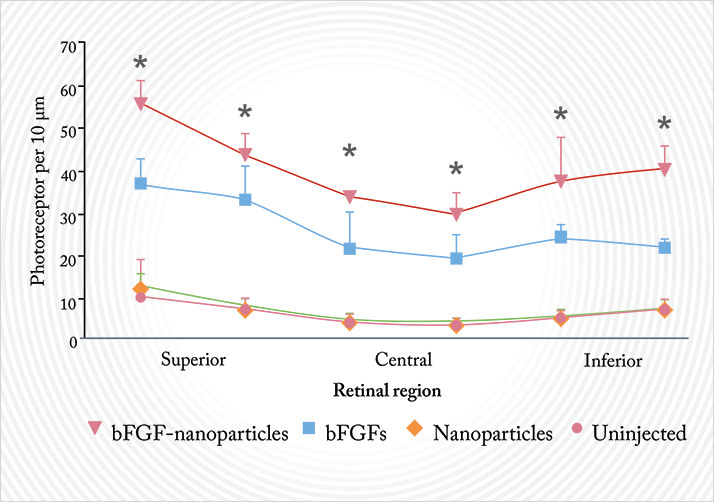
So far, I have shown you nanoparticles that are acting at the front of the eye. But is there any evidence that nanoparticle formulations of drugs might make a difference at the back of the eye, at the retina? Based on preclinical studies in rats, the answer to that is yes. Royal College of Surgeons (RCS) rats are a commonly-used animal model of retinal degeneration, and it’s long-established that intravitreally-injected basic fibroblast growth factor (bFGF) exerts a protective effect on the retina in these mice, delaying photoreceptor degeneration. But the effect is as short-lived as bFGF’s half-life; you really need continual drug delivery to protect the retina in any meaningful way.Gene therapy with viral vectors is one method – although this is not without risks, like unwanted immune system reactions or insertional mutagenesis. The ideal alternative would be a delivery system that gets an effective amount of bFGF to the target site and gives sustained drug – or gene plasmid – release without causing serious complications. Again, gelatin nanoparticles are a proposed solution to this problem. A group of researchers in Japan created 125I-radiolabelled gelatin nanoparticles, injected them intravitreally into the eyes of RCS rats, and measured how long the radioactivity persisted for, as a marker of how long bFGF-containing nanoparticles could persist at the retina (7). The answer: at least 30 days. They then went on to intravitreally-inject bFGF-containing nanoparticles into the RCS mice and determined the photoreceptor density of these mice eight weeks later (Figure 7). RCS rats injected with bFGF nanoparticles retained a significantly greater photoreceptor density than all of the other treatment or control groups (7). This has worked with DNA too – an Oklahoma-based group has used PEG-and-peptide nanoparticles to deliver plasmids into the retina, achieving gene expression and impressive improvements in the phenotype of genetic mutant mice with a retinitis pigmentosa-like phenotype (8). As promising as these studies appear, they are still using intravitreal injections – something that, ideally, we want to avoid.
Eye drops for retinal disease
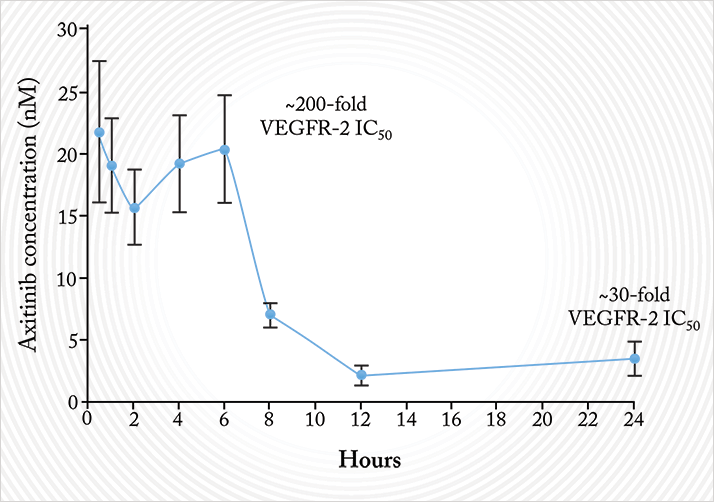
Earlier, I told you that nanoparticles had the potential to deliver topically-administered drugs to the back of the eye. Well, that’s been done too. Axitinib is a small-molecule receptor tyrosine kinase inhibitor. Like ranibizumab, it inhibits VEGF signaling. Like Fovista, it also inhibits platelet-derived growth factor (PDGF) signaling. Unlike both, it also inhibits c-kit, a survival factor for developing blood vessels. Unlike both, it also has a half-life of a few hours, whereas ranibizumab and aflibercept each have half-lives of several days in the human eye. This means that axitinib needs to be dosed regularly – perhaps even daily – to exert its therapeutic effect on the retina. Clearly, daily intravitreal injections are not an option. Could a topically administered nanoparticle formulation be the answer?Hongming’s team formulated axitinib molecules into the aforementioned MPPs in an attempt to do so. They wanted to establish if a topically administered axitinib-MPP formulation could get therapeutic doses of the drug to the retina. Their first preclinical pharmacokinetic analyses were performed in the eyes of rabbits, and what they found was clear: compared with the control of regular axitinib applied topically to the eye, topical axitinib-MPP resulted in a fivefold greater retinal drug exposure (9). The fact that they got the drug to the retina is one thing – but were these therapeutic doses? Ranibizumab exerts most of its action by binding VEGF, thus preventing its binding to VEGF receptors. Axitinib’s IC50 – the concentration at which a drug antagonizes 50% of its target receptors – for a key VEGF receptor (VEGFR-2) is 0.1 nM. A single topical 2% axitinib-MPP administration resulted in axitinib concentrations that were between 10 and 200 times greater than its VEGFR-2 IC50 in rabbits (Figure 8). No irritation was observed with the axitinib-MPP eye drops – a good sign if you’re proposing regular topical administration to the eye – and experiments that compared axitinib-MPP eye drops with intravitreally-administered bevacizumab (in a rabbit retinal vascular permeability model) showed that both drugs significantly and similarly improved vascular leakage (Figure 9) – confirming that therapeutic concentrations were achieved with the topical administration of axitinib-MPPs.
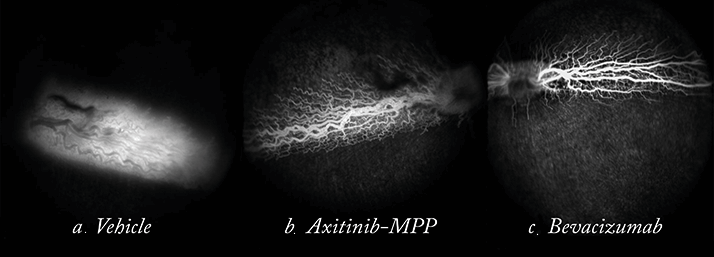
Hongming and her team are currently evaluating other small-molecule receptor tyrosine kinase inhibitors – but the principle has been proven. Nanoparticle technology allows one to topically deliver drugs to the posterior-segment, at least in rabbits. Would it be premature, then, to imagine a future where the frequency of intravitreal injections is significantly reduced?
Imagine the potential
Medicine has already begun to see significant progress driven by nanotechnology, but its tremendous promise is only just beginning to be realized: improved drug delivery really is just the tip of the iceberg as to what modern technologies like nanotechnology will achieve over the next ten, twenty or thirty years. Just imagine being able to prescribe eye drops for wet AMD: rather than having clinics bursting-at-the-seams with patients requiring their monthly anti-VEGF injection, you’ll just need to see them for their regular checkups instead. Imagine the impact that better eye drop regimen compliance and fewer drug-related adverse events could make to patients with glaucoma or ocular surface disease. Imagine what localized and specific gene delivery could do for patients with inherited vision disorders. The application of materials science and chemical engineering to just one aspect of medicine – drug formulation – is just one of many ways nanotechnology can transform ophthalmology. But if you’re looking at the big picture, think small – really small – about how you can improve things.Robert Langer is a chemical engineer, the David H. Koch Institute Professor at the Massachusetts Institute of Technology, author of over 1,250 publications, owner of over 1,000 patents, and the most cited engineer of all time. Justin Hanes is the Lewis J. Ort Professor at Johns Hopkins, where he holds appointments in the Schools of Medicine, Engineering, and Public Health, and where he directs the Center for Nanomedicine at the Wilmer Eye Institute. The MPP technology was discovered in his lab. Hongming Chen is the Chief Scientific Officer at Kala Pharmaceuticals, Inc. Kala is the licensee of the MPP technology and is developing the technology for applications in various mucosal organs including the eye.
References
- L.M. Ensign, C. Schneider, J.S. Suk, et al., “Mucus penetrating nanoparticles: Biophysical tool and method of drug and gene delivery”, Adv. Mater., 24, 3887–3894 (2012). doi: 10.1002/adma.201201800. S.K. Lai, Y.-Y. Wang, J. Hanes, “Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues”, Adv. Drug Deliv. Rev., 61, 158–171 (2009). doi: 10.1016/j.addr.2008.11.002. K. Brazzell, L.R. Schopf, E. Enlow, “Evaluation of Pharmacokinetic Profile of Novel Ophthalmic Formulation of Loteprednol Etabonate”, Paper presented at: The American Society of Cataract and Refractive Surgery/ASOA Symposium and Congress; April 25, 2014; Boston, MA. https://ascrs.confex.com/ascrs/14am/webprogram/Paper4253.html. S. Karen, “The impact of medication regimen factors on adherence to chronic treatment: a review of literature”, J. Behav. Med., 31, 213–224 (2008). doi: 10.1007/s10865-007-9147-y. R. Chen, Y. Qian, R. Li, et al., “Methazolamide Calcium Phosphate in an Ocular Delivery System”, Yakugaku Zasshi, 130, 419–424 (2010). L. Contreras-Ruiz, G.K. Zorzi, D. Hileeto, et al., “A nanomedicine to treat ocular surface inflammation: performance on an experimental dry eye murine model”, Gene Therapy, 20, 467–477 (2013). doi: 10.1038/gt.2012.56. T. Sukat, N. Kuno, F. Takamatsu, et al., “Prolonged protective effects of basic fibroblast growth factor-impregnated nanoparticles in Royal College of Surgeon Rats”, Invest. Ophthamol. Vis. Sci., 48, 3381–3387 (2007). X. Cai, S.M. Conley, Z. Nash, et al. “Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa”, FASEB J., 24, 1178–1191 (2010). doi: 10.1096/fj.09-139147. L. Schopf, E. Enlow, A. Popov, et al., “Enhanced topical delivery of a small molecule receptor tyrosine kinase inhibitor (RTKi) via mucosal-penetrating particle technology”, Invest. Ophthalmol. Vis. Sci., 54, E-Abstract 1087 (2013).
