How did the concept of light adjustability arise?
The selection of IOL power for cataract surgery is not an exact science. Inaccuracies in biometry and the unpredictability of effective lens position and wound healing often result in residual refractive errors and unsatisfactory visual outcomes for patients. The rationale for the development of the Light Adjustable Lens was to address these important issues, given that cataract removal is one of the most commonly practiced surgeries in the world. The project started in 1997 with a collaboration by Daniel Schwartz from the University of California, San Francisco, and Robert Grubbs, Chemistry Professor at the California Institute of Technology. The objective? The creation of a biocompatible lens that could be safely and non-invasively reshaped with a laser after surgery to correct myopic, hyperopic and astigmatic refractive errors.How does the technology work?
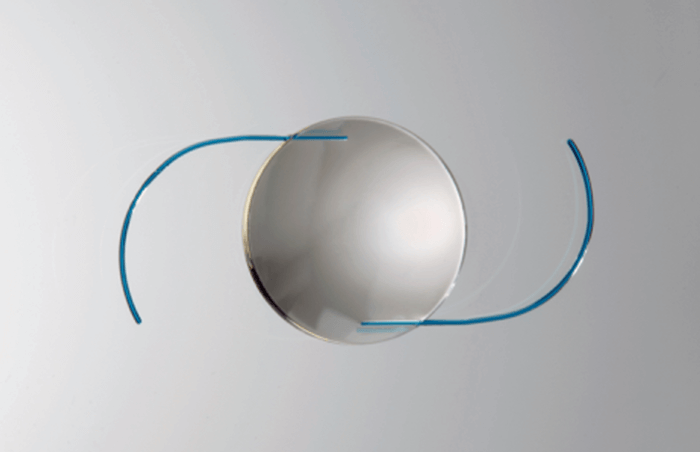
The Light Adjustable Lens is implanted using standard surgical techniques for conventional cataract surgery. After the eye has healed, the patient comes in for a routine vision exam. The surgeon can then customize the lens power by directing a low intensity beam of UV light onto the lens from outside the eye. The light is delivered via the office-based light delivery device (LDD; RxSight), and the special photosensitive material of the lens reacts to the UV light and changes shape to match the prescription the patient selected during their eye exam. Multiple adjustments can be made to ensure the best result prior to making the changes permanent.How does it feel to be involved with the first approved adjustable lens technology?
We are incredibly grateful to all the patients, surgeons, medical staff, study teams, scientists and employees who worked to deliver the technology. After all, a great deal of work – nearly two decades – has gone into the research, development and approval of the light adjustable and light delivery technologies. Though there are many advanced IOLs on the market and in development, we believe we are in a good position as the only approved IOL technology that can be non-invasively adjusted after implantation.By Vance Thompson, Founder of Vance Thompson Vision, Sioux Falls, SD, USA The RxSight Light Adjustable Lens is the only FDA-approved IOL that can be customized after implantation in the patient’s eye – and that’s what I love about it. Being able to adjust the lens power postoperatively can overcome many of the healing issues that limit refractive accuracy – such as effective lens position, posterior corneal astigmatism, and incisional healing issues that can increase or decrease astigmatism. When a patient truly understands how implant measurements and calculations are performed preoperatively – and that certain aspects (such as effective lens position) are an “estimate” – I have found that they really appreciate the idea of a lens implant that uses modern-day formulas but can be adjusted in their eye. A lens that is truly customized and individualized to their life vision needs. The technology is a paradigm shift in cataract surgery because it will help overcome the predictive limitations that all surgeons struggle with. Currently, we have to try to ‘paint’ pictures with words preoperatively for the blurry cataract patient on their vision options. We can’t truly show them what their options are as we would do in contact lens fittings and before refractive surgery, because their cataracts and blurry vision will not allow such testing. But being able to adjust the power with the Light Adjustable Lens means we can simulate various refractive options and adjust their power to the desired correction. We can also perform another adjustment if they so desire – for example, more powerful near vision – and when they are satisfied with their final vision, we can lock it in so they can enjoy that implant power for the rest of their life.