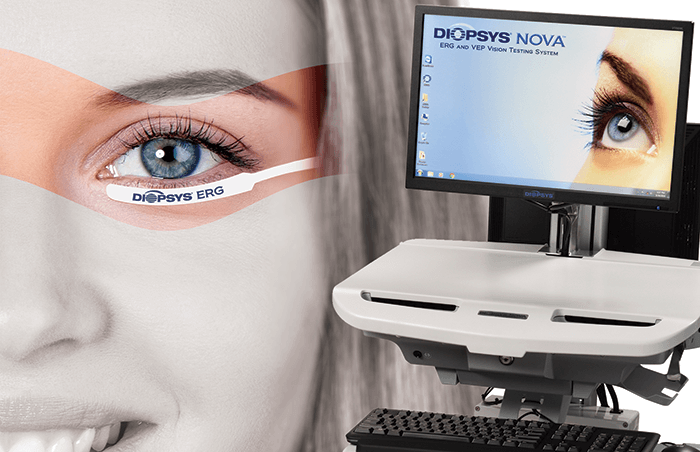
Electroretinography (ERG) provides an objective measurement of retinal function and is especially useful in detecting glaucoma. How? By identifying “stressed” retinal ganglion cells at a subclinical stage, when the cells have become dysfunctional, but are still alive. To put its value into context, a reduction in pattern ERG signal in glaucoma suspects has been shown to precede structural changes to the retinal nerve fiber layer by eight years – so this represents a huge window for intervention before permanent damage occurs.
But can you efficiently use this technology? ERG instruments have historically been expensive and cumbersome, and the test difficult to perform and interpret. In response to these challenges, Diopsys created an accessible, in-office visual electrophysiology suite, including ERG, VEP, and ffERG vision tests.
To overcome the device and result interpretation issues, “We developed two different practice-friendly testing platforms, the Diopsys NOVA cart system, and the Diopsys ARGOS tabletop system. And through our extensive clinical research, we can now provide ophthalmologists with clear test results that are color-coded based on documented reference ranges”, explains Joseph Fontanetta, Diopsys CEO.
Another hurdle that the developers had to overcome was that traditional ERG requires sensors that make contact with the cornea – typically a contact lens or an electrode placed directly on the eye. This both risks damage to the cornea and causes patients discomfort, jeopardizing patient compliance and quality test results. Some ophthalmologists have turned to generic skin electrodes, but these sensors cover a large surface area, and come into contact with the facial muscles, often contaminating test results by picking up excess electrical energy. By developing a small external sensor that is placed under the eye, the Diopsys development team avoided these problems.
“We wanted to make this important vision test a practical, everyday diagnostic tool for ophthalmologists and their patients to help diagnose disease earlier and enhance patient management,” says Fontanetta, “so we took the same beneficial ERG vision testing found in large research institutions, and made it accessible to eye care practices all over the world.”
Availability varies globally.

Treating AMD is always a challenge. But although wet AMD can be managed (albeit with frequent appointments and costly injections), for dry AMD the situation is even bleaker. Currently, the only options are vitamin supplements and telescopic optics. But the lenses are large (requiring a 7 or 8 mm incision), and the surgery is time-consuming and associated with extracapsular type complications. One ophthalmologist felt they simply weren’t good enough – so he decided the only solution was to make his own.
“The biggest unmet ophthalmic need in the world right now is dry AMD, and we have no suitable treatments. I’ve implanted many telescopic lenses for AMD, and you have to put several things into the eye, then fix them together like Lego. It’s a nightmare. Developing a new IOL for AMD was the greatest opportunity I could see. The first iteration of my design, iolAMD, was encouraging – it’s foldable and can be inserted through a small incision. The total procedure, including cataract extraction, takes less than 10 minutes,” says Bobby Qureshi, founder and CEO of LEH Pharma.
The EyeMax Mono is the successor to iolAMD. A single lens system with patented optics never used before in any IOL – it’s the world’s first (and currently only) extended macular lens. Like its predecessor, it’s much less bulky than other offerings, and was designed to be as simple to implant as a typical monofocal, with a recovery period much shorter than for a normal telescopic IOL – a matter of weeks, rather
than months.
So how does it work? “The EyeMax is completely unique, and represents a whole new method of improving vision in AMD patients. It creates a high quality image across 15 degrees of the macula, and magnifies this image by 20 to 30 percent,” explains Qureshi. The theory behind the lens is that using innovative new optics, the patient should be able to achieve the visual potential of any residual part of the macula, and can change their preferred retinal locus as the AMD progresses – and if implanted binocularly, it will help the visual cortex to fill in the central visual field using the healthy areas of the macula from both eyes, creating a compound image – further work is being done to evaluate this effect.
The EyeMax has now been implanted in over 1,000 eyes, with studies showing a mean gain of at least two, and in some studies as many as five, ETDRS lines for distance and reading; some studies soon to be published also show significant improvement in reading speed. More studies are now underway to better understand which patients will benefit – although Qureshi envisions the technology being a possible choice for any patient with macular disease as an alternative to a monofocal. There are also plans to submit the lens for FDA approval in 2017.
“For the first time, surgeons can offer something for this very large population of patients that may be able to restore some vision, without the downsides of complicated, risky surgery, or compromises such as one eye needing to adapt after surgery, or a reduction in visual field,” says Qureshi. “This lens is suitable for patients with early and intermediate AMD, and continues to work even as the disease progresses – something no other option can offer,” he adds. As for the future of the lens, a sulcus variant for pseudophakic patients is expected to launch in 2017. Qureshi also aims to further refine and improve upon the EyeMax to expand the current patient selection criteria – and it’s already being used by some surgeons in patients with diabetic maculopathy, epiretinal membranes, and other macular disorders. There are other plans in the pipeline too, including bespoke spectacle optics, and laser treatments for AMD. “This IOL is the culmination of almost a decade of work, that was originally inspired by my own experience in implanting thousands of telescopic implants of every kind, and an optical error in the Hubble telescope. I feel it could be one of the greatest innovations of recent times – and now, it’s finally going to market,” says Qureshi. The EyeMax Mono will be available in the EU in 2017, and is currently unavailable in the US or Japan.

When you’re working out where a company is going, it helps to look at where it has been. HAAG-STREIT SURGICAL can trace its roots back over 150 years, to when Johann Diedrich Möller began producing high-precision optical components in Wedel, Germany. New technology was always embraced, such as products which included combinations of binoculars and cameras, and also anamorphotic lenses used in film projectors. In 1963, Möller Wedel produced the world’s first ceiling-mounted microscope for use in either micro surgery or ophthalmology, and entered the microsurgical field. In the 1990s it became part of HAAG-STREIT Group, and continued to focus on innovation in ophthalmology. The next major milestone was the launch of the world’s first intraoperative OCT (iOCT®) device, to provide noninvasive imaging of structures and layers within the tissue of the eye during surgery – a technology HAAG-STREIT SURGICAL is now continuously working to improve and refine. According to the HAAG-STREIT SURGICAL team, “understanding the needs of physicians is our main source of inspiration. By thinking beyond today’s standards and looking at what the next unmet need will be, we work on solving surgical challenges and improving medical workflows.”

The Kahook Dual Blade (KDB) wasn’t originally created for treating glaucoma. The aim of its creator, Malik Kahook, was to remove a section of trabecular meshwork (TM), intact, for imaging studies – but he quickly realized that he had created a device that solved an unmet need in surgical glaucoma: the ability to remove TM without damaging adjacent tissue. So, what makes the device different? The tip provides controlled entry into Schlemm’s canal; a ramp lifts the TM as the device is advanced, stretching the tissue before it’s cut by the dual blades. These precise, parallel incisions leave behind wide open canal space for aqueous to flow into the collector channels. “In the past, attempts to open up flow channels at the level of the TM centered on single incisions, or on ablating tissue – causing collateral damage and only partially removing the TM. More complete removal with a simple but elegant surgical device is a practical and cost-effective solution,” explains Yasir Iqbal, New World Medical’s Marketing Director. “The KDB is versatile; it can be combined with cataract surgery, or used in a standalone procedure, and it gives surgeons a single tool they can use to bypass the TM with confidence,” he adds.
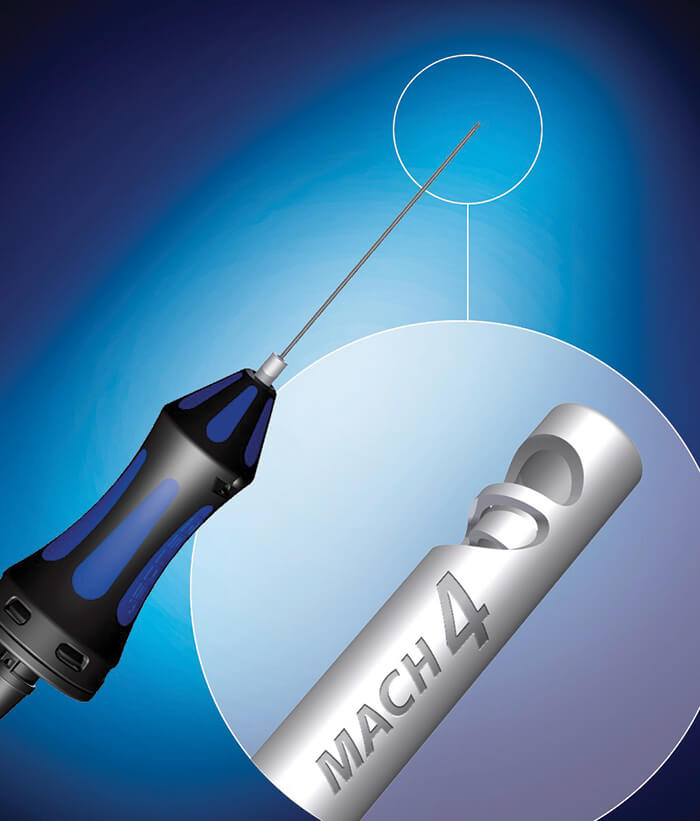
Traditional vitrectors do the job – but they can always be made better: to cut and aspirate more effectively, cause less traction on the retina and make the action more discrete, i.e. closer to the aspiration port. To do that, you have to cut faster. In 2014, Geuder introduced the MACH2 double-blade vitreous cutter. It could perform up to 12,000 cuts per minute (cpm), made two cuts per work step, and the aspiration window was permanently open – making for faster core vitrectomy, with fine control and a delicate action. How could that be improved? A doubling. The MACH4 vitrector features four blades and cuts up to 24,000 cpm. Above and beyond MACH2, it’s designed to reduce traction, increase safety, and makes for precisely controlled shaving. It’s faster and more efficient at vitreous aspiration, causes even less mechanical stress on the retina (leaving it virtually immobile). Like MACH2, the port remains permanently open, rendering duty cycle management obsolete and giving a constant aspiration flow and controlled shaving. “We believe surgical procedures need to be constantly improved and updated in order to minimize risks and complications”, says Hamadi El-Ayari, Geuder Sales and Marketing Vice President. Currently in clinical testing. Not available in the US.
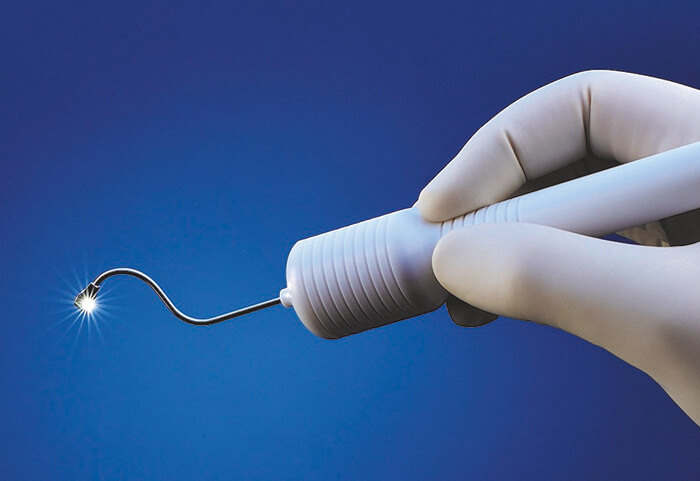
A leading cause of blindness, wet AMD, can be managed – but doing so is a hefty burden. The time and cost of the monthly anti-VEGF injections is considerable to patients and healthcare systems alike – and worse, only 34 percent of patients respond with improved vision. SalutarisMD aims to reduce that treatment regimen and address the unmet need. What do they propose? Minimally invasive brachytherapy – an outpatient procedure that can be performed in 15 minutes. A single-use, sterile applicator is used to place the therapeutic radioisotope behind the eye, adjacent to the area requiring treatment. Nothing is left behind and the intraocular space isn’t violated. “A small clinical study has produced encouraging patient outcomes, including visual improvements and absence of the pathologic lesion, with no additional interventions for two years,” says Laurence Marsteller, CEO of SalutarisMD, “and new clinical trials are currently planned at the University of Arizona and Moorfields Eye Hospital.” He hopes that “in the future, SalutarisMD technology can offer a new treatment option for wet AMD patients.” Caution: Currently limited to investigational use only.
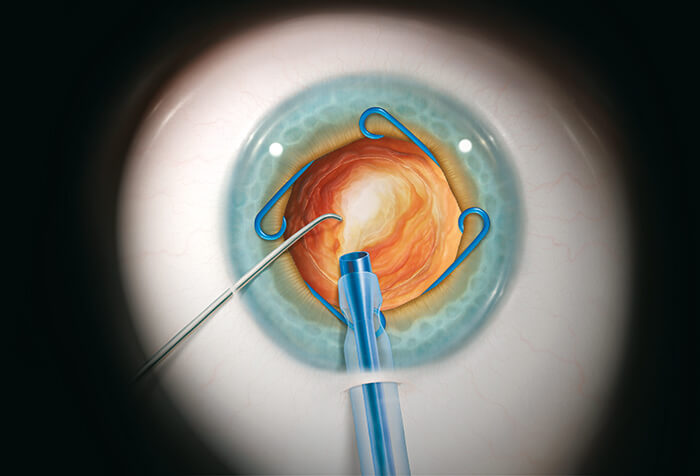
Partnering with surgeons to make their ideas a reality is the fastest way to foster innovation. Here’s one example. Small pupils and intraoperative floppy iris syndrome limit visibility – and access – during cataract procedures. If there’s no safe way to expand the pupil, the potential for complications skyrockets. Boris Malyugin’s idea: the Malyugin ring, a pupil expander that’s gentler and easier to use than iris retractor hooks. MicroSurgical Technology (MST) partnered with Boris to develop and commercialize his eponymous ring, and has helped to make challenging small pupil cataract cases safer and more routine. Since it was launched onto the market over nine years ago, the Malyugin Ring has been continually modified and improved – for example, the Osher modification to the injector enabled easier release and re-engagement of the ring. The Malyugin Ring 2.0 was released in May 2016 – an updated version that is even gentler on the iris and even easier to use. Today, over one million Malyugin Rings have been used in cataract surgeries around the world. MST is dedicated to solving clinical problems in partnership with some of the most prominent ophthalmologists in the world: Ike Ahmed, Bobby Osher, David Chang, and (of course) Boris Malyugin. For challenging procedures such as IOL exchange, scleral fixation of an IOL, and iris repair, MST developed their anterior segment micro-instrumentation for safer management of complicated surgery. “These instruments could be described as the fire extinguishers on the wall: good to have in the event of complications,” says Jeff Castillo, Company President. One newer innovation is the Allegro silicone I/A system. “Its complete silicone coverage and unique geometry help provide a safer, more precise way to remove cortical material, while also providing excellent sub-incisional access,” says Castillo. “Our Allegro system is a revolutionary change to the I/A stage of cataract removal”, he adds. The first generation of Allegro was released in 2015, with the next generation to hit the market in 2017. “MST is always looking to partner with surgeons with ground-breaking ideas,” says Castillo, “and we will continue to develop products inspired by ophthalmologists, in order to solve clinical problems and help to provide the best patient outcomes possible.”
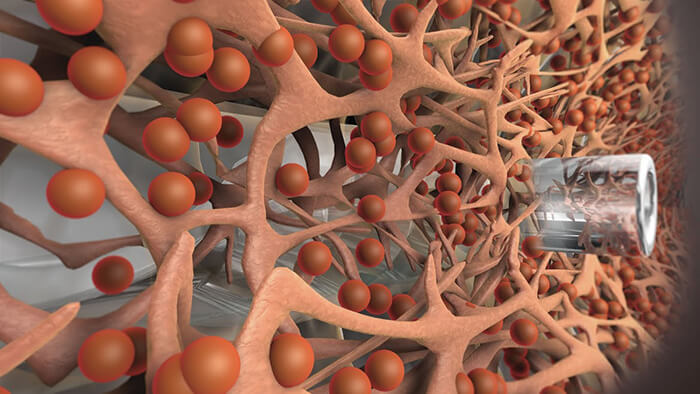
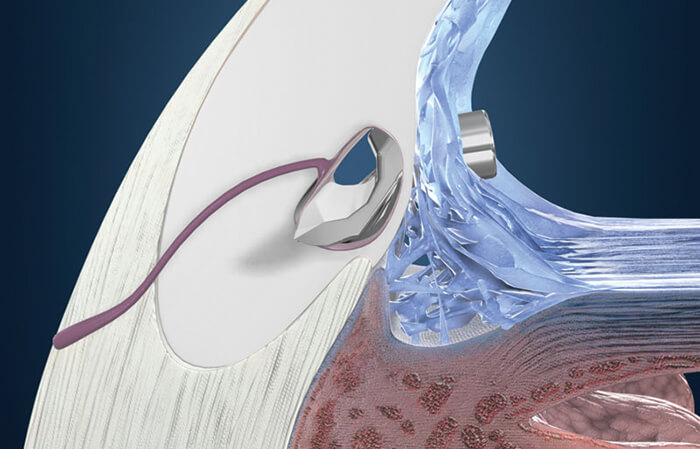
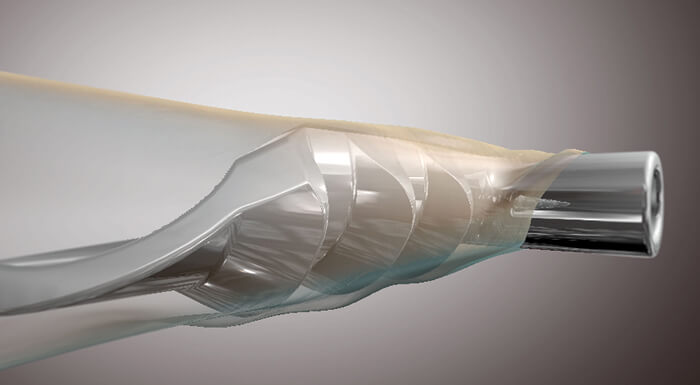

Glaucoma is an area of ophthalmology that has become a hotbed of innovation in recent years. As a leading cause of blindness worldwide with limited treatment options, it isn’t hard to see why. The disease is often treated by administering eyedrops to control IOP, although many patients struggle to adhere to their sometimes oppressive regimens. For patients whose IOP cannot be controlled with medication, surgery is often the next step. But traditional surgeries, such as trabeculectomy, are invasive and come with a significant risk of adverse events, or failure. This was the challenge that glaucoma surgeons faced – until the advent of microinvasive glaucoma surgery (MIGS). Today, MIGS is changing the way that glaucoma is treated. But not so long ago, the term didn’t even exist. Enter Glaukos: it took the company over 10 years of research, testing and commercialization to bring its idea to market, and it all began when life sciences investor Olav Bergheim brought a family member with glaucoma to meet ophthalmologist Rick Hill for an assessment. Rick told Olav’s relative that he had advanced glaucoma, and that he required bilateral trabeculectomies. But Olav thought there had to be a better way – and Rick put forward his idea of a trabecular bypass with an internal approach, using a stent small enough to maintain the bypass and restore outflow. However, Rick had been advised that the technology simply didn’t exist to manufacture the tiny stent with enough precision. Olav wasn’t so sure, and he brought in a fluid dynamics expert to work on the idea. Together, the three men created the concept for the first iStent® prototype. But commercializing a medical device that established an entirely new category of glaucoma surgery was never going to be easy. “Our first major challenge was the development of the microstent implant and procedure. At that time, producing devices of this size challenged the limits of micromachining. Our next big challenge was establishing a regulatory path for our company, and the emerging category of MIGS – a daunting task, which involved working with the FDA to figure out how the iStent could be evaluated for safety and efficacy,” explains Thomas Burns, CEO. Establishing a new class of treatment meant the company was subject to a high level of scrutiny from regulatory bodies, and had to work to present the benefits of its product to glaucoma surgeons.
Now that the device is on the market, the company is working to bring the iStent to a wider audience, and to provide support and education for surgeons who wish to introduce it to their own practices. Its mission, explains Burns, is “to lead the global glaucoma market, and advance the existing standard of care.” “We pioneered MIGS in order to revolutionize the traditional glaucoma treatment and management paradigms. The first prototype of the iStent was made in 1999, and launched in the United States in 2012 – and to our knowledge, it was the smallest medical device ever approved by the FDA. The treatment of glaucoma is our sole focus, and we now have 55 peer-reviewed articles published on iStent, long-term clinical results and over 200,000 implanted globally,” says Burns. Looking further ahead, Glaukos plans to use its existing platform technology to build a portfolio of injectable, microscale therapies for the treatment of the complete range of glaucoma disease states. With its next generation product, the iStent inject®, it envisions transitioning to an injectable therapy. iStent inject is designed to further reduce intraocular pressure by potentially delivering multiple stents into the trabecular meshwork through a straightforward click-and-release motion. An extended drug delivery and implantable platform, named iDose®, is in clinical trials and is designed to deliver months of prostaglandin therapy for glaucoma management – tackling the ubiquitous issue of non-adherence to medication. “There are now more options available for glaucoma patients than in the past,” says Burns, “and defining them helps both ophthalmologists and the wider ophthalmic community to administer treatment. Early detection and treatment of glaucoma is key to the long-term well-being of patients, and we aim to remain one of the leaders in this area.”
