
Knowing and understanding your patients’ corneal biomechanics can be invaluable. It helps with assessing ectasia risk, diagnosing keratoconus, measuring the effects of corneal collagen cross-linking (CXL), and even glaucoma prediction and management. The OCULUS Corvis ST combines a tonometer with an ultra-high-speed Scheimpflug camera, to not only measure IOP, but also provide a detailed assessment of corneal biomechanics. How does it work? By applying an air pulse and simultaneously monitoring the cornea’s response, and capturing 140 images of the horizontal sectional plane of the cornea, within just 31 ms. “This information has helped transform our understanding of the eye’s anterior segment. One example is biomechanical-corrected IOP. We know that corneal thickness, age and the biomechanical response of the cornea can affect IOP readings taken by applanation tonometry, and that these factors need to be corrected for,” says Sven Reisdorf, Corvis ST product manager. The Corvis ST measures both biomechanical response and corneal thickness with high precision and corrects for both, giving ophthalmologists more accurate IOP readings, and with it, better guidance for making treatment decisions. “The Corvis ST is also helping to improve the detection of corneal ectasia – the Vinciguerra Screening Report software combines biomechanical information with pachymetric progression data to generate the Corvis Biomechanical Index (CBI) – and presents these results in comparison with normative values in easy-to-read graphs, making for swift and easy keratoconus detection”, adds Reisdorf. Finally, you can combine topographic and tomographic data from the Pentacam with biomechanical data from the Corvis ST to produce the Tomographic Biomechanical Index (TBI) – an artificial intelligence approach that helps improve the detection of patients with a significant risk for developing ectasia after refractive surgery. The hardware was developed by OCULUS head of R&D, Andreas Steinmüller, and by engineer Matthias Krug. The analysis software was produced in collaboration with leading researchers, including Renato Ambrósio Jr., Ahmed Elsheikh, Paolo Vinciguerra and Cynthia Roberts. “Currently, the most important application for the technology is in determining ectasia risk after refractive surgery – we can identify corneas predisposed to the condition by combining the biomechanical information with tomographic data measured by the OCULUS Pentacam, which gives us a truly unique analysis,” explains Reisdorf. Availability of the product and features may vary by country. Currently not for sale in the US.
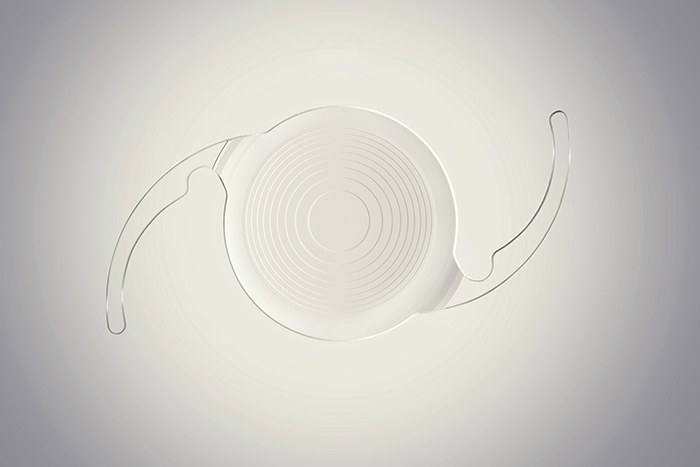
Everyone wants to offer an IOL that leaves patients spectacle-free after cataract surgery – and that involves making multifocal IOLs. However, multifocality is an optical compromise, and risks glare, halo and loss of distance contrast sensitivity. The name of the game is building a better multifocal lens, with the objective of offering the best-possible range of vision with the fewest visual disturbances. In 2009, Abbott’s R&D team started work on an IOL design that took a completely different approach to other multifocal IOLs. The objective was to offer patients a full range of high-quality vision (enabling less frequent spectacle use), a low incidence of halos and glare, and distance contrast vision comparable to that of monofocal lenses. Eschewing the traditional multifocal IOL approach of splitting light between near and distance focal points, their IOL employs a proprietary echelette design, which creates a novel pattern of light diffraction that elongates the focus of the eye and extends the range of vision. This is combined with achromatic technology to correct chromatic aberration and enhance both image contrast and quality of vision. What resulted was the Tecnis Symfony and Tecnis Symfony Toric IOLs; the first extended depth of focus (EDOF) presbyopia-correcting lenses for use in patients with and without astigmatism. The lenses have now been evaluated in multiple studies in over 2,000 eyes across the world, including a US clinical trial. Results show continuous high quality vision at all distances, a low incidence of halo and glare, and a minimization of chromatic aberration. Tecnis was able to deliver 20/20 vision even in the presence of up to 1.5 D of astigmatism, and in one questionnaire, 85 percent of respondents reported being completely or almost completely spectacle-free. Furthermore, as Symfony’s EDOF vision is independent of pupil diameter, this allows for good performance under all light conditions. “These lenses provide a new option for patients that may result in better vision across a broad range of distances. Patient satisfaction has been high, with over 94 percent of patients saying they would recommend them to their friends and family,” says Leonard Borrmann, head of R&D at Abbott. “By building on our successes so far, we plan to continue to create innovative IOLs,” he adds.
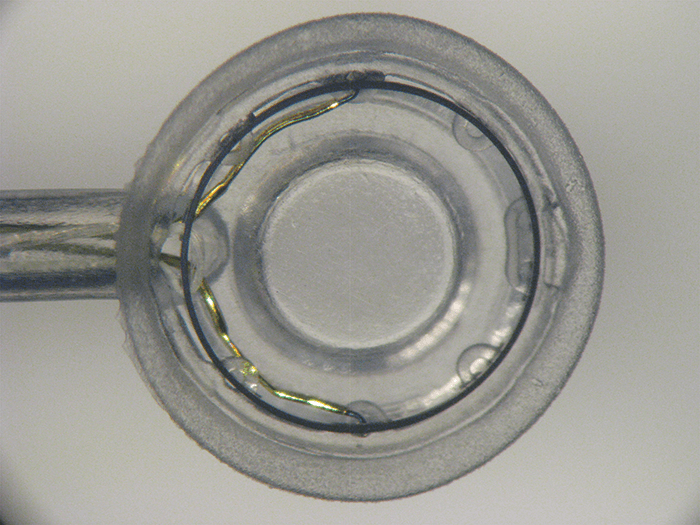
The creation of a consistently sized, round and well-centered capsulotomy opening in the lens capsule is perhaps the most challenging step of cataract surgery. Manual capsulotomies using forceps can be tricky, and depend strongly on the surgeon’s skill level. Creating your capsulotomy with a femtosecond laser is another option – if you can afford the expensive equipment, and don’t mind the extra time or potential complications. What if there was another alternative that offered a convenient automated option without the hefty price tag? Christopher Keller and David Sretavan were both new to medical device development when they began work on a miniature automated capsulotomy device. Christopher had a background in microelectromechanical systems, and created gizmos such as micro-knives to perform surgery on individual nerve cells grown in petri dishes. David became a customer, and it wasn’t long before the pair decided to enter the world of medical device development. With no money for fancy lasers, they were instead inspired by the elegance of squid suction cups that create a complete force circuit – and leveraged this concept into their own solution to the capsulotomy conundrum: Zepto. The disposable Zepto device consists of a suction cup that holds the capsule and lens steady while pulling them against the tiny nitinol capsulotomy ring. A four millisecond electrical pulse train runs through the nitinol ring to neatly create the capsulotomy without damaging neighboring tissues. This combination of suction and an optimized pulse train to instantaneously create all 360 degrees of the capsulotomy is unique to Zepto.
Initial clinical studies were promising. One study involving over 60 patients in El Salvador, where access to cataract surgery is limited, demonstrated Zepto’s effectiveness even in challenging cases such as poorly dilated pupils, hard cataracts and zonular pathology. Even without some of the instruments and devices normally used in such cases, all Zepto capsulotomies were successful and delivered good outcomes for the patients. “Zepto can quickly and consistently produce a round capsulotomy with an edge exhibiting excellent tear strength,” says John Hendrick, CEO of Mynosys Cellular Devices, “we believe that no matter what kind of cataract you’re looking at, or how tricky the case, Zepto can make the procedure safer and easier for the surgeon.” The Zepto silicone suction cup offers another big advantage – it essentially disappears once inside the eye, allowing the surgeon to use patient fixation and Purkinje images for intraoperative centration of the Zepto capsulotomy precisely on the patient’s visual axis. This is especially useful for surgeons using premium IOLs. Furthermore, a well-constructed, strong capsulotomy edge that is perfectly centered on the visual axis will be critical for next generation IOLs that are fixed to the capsulotomy edge. The ability for surgeons at all skill levels to automatically and consistently produce round and strong capsulotomies will benefit all patients. This, combined with personalized visual axis centration makes Zepto unique and underlies Zepto-assisted cataract surgery (or ZACS). The low cost of ZACS makes it an attractive alternative to the more expensive femtosecond laser, which is out of reach for millions of patients around the world. “Zepto puts a great capsulotomy in the hands of all surgeons – the consistent roundness and sizing ensures more effective lens positioning, to benefit visual outcome for patients,” explains Hendrick. As an easy to use and highly effective alternative to the femtosecond laser, Zepto has the potential to be a truly disruptive force in cataract surgery. It is easy to see how Zepto alters the cataract surgery landscape in developed markets, either as a stand-alone technology or integrated into a phaco machine. However, there is also enormous interest from the fast growing international markets where challenging cases are more frequent, and Zepto is beginning to experience tremendous growth in the premium segment. “With ZACs, surgeons can create perfect capsulotomies in both simple and complex cases, whether using monofocals or the latest premium lenses. As Zepto integrates seamlessly into cataract surgery, it doesn’t interfere with the surgeon’s normal routine, or their patient flow – instead of reaching for capsulorhexis forceps, they simply reach for Zepto,” adds Hendrick. The Zepto device is CE marked, but is not yet available in the US.
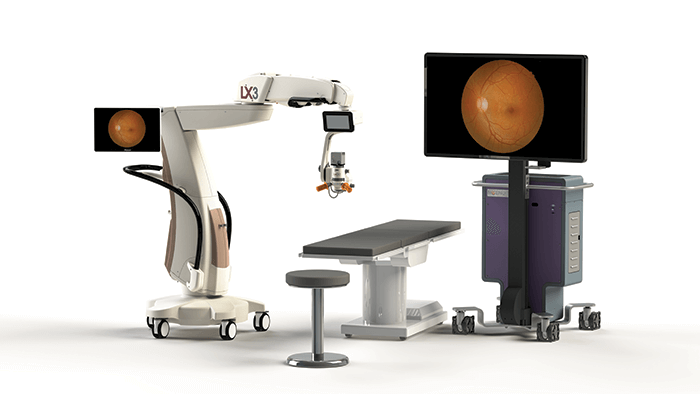
For retinal surgeons, visualization can be a major issue in the operating theater. Opacities and aberrations of the eye, shadows, glare from fiber optic illumination, poor access when navigating around the crystalline lens – there can be a whole host of visual barriers between the surgeon and the tissue they’re working on. What does this mean in practice? Hours hunched over a microscope – an instrument that’s never been known for having good ergonomics – which can add up to both muscle pain and fatigue. Alcon, in collaboration with TrueVision 3D Surgical, believes it has designed a product to end those woes in the form of its NGENUITY® 3D Visualization System, a platform designed to digitally assist vitreoretinal surgery, and enhance visualization of the back of the eye with the aim of improving the surgeon’s experience. It consists of a 3D stereoscopic high-definition digital video camera and workstation, and acts as an accompaniment to the surgical microscope, displaying real-time or images from recordings. The NGENUITY® 3D Visualization System allows retinal surgeons to operate looking at a high definition 3D screen, instead of bending their necks to look through the eye-piece of a microscope. The high dynamic range camera provides high resolution, image depth, clarity and color contrast, and the 3D view allows depth perception not previously available on standard television models often used in the OR. Viewing options include increasing magnification but retaining a wide field of view, and using digital filters to customize the view during each procedure (1, 2). The system can also be used to highlight ocular structures and tissue layers. Engineered with a specific focus on minimizing light exposure to the eye (2), it can be used under lower illumination, and remember, the NGENUITY® display means less time at the microscope – which could help improve posture. Broadcasting the surgery on a larger screen also means that the operating team can see exactly what the surgeon sees, in real-time. “The NGENUITY® 3D Visualization System takes vitreoretinal surgery to a more intuitive operating experience, offering greater depth and detail during surgery,” said Mike Ball, CEO of Alcon. “Our goal is to provide surgeons with better visualization, facilitate teaching, and ultimately improve patient outcomes,” he adds. The NGENUITY® is available in the US and most EU countries, with further launches planned over the course of 2017.

One of the defining aspects of research is that it never stops. We might be in the 25th year of OCT, but the advances over that period have been relentless: Time-Domain OCT shifted to Spectral-Domain; we’re now entering the era of Swept-Source (SS) OCT, and of course, you’re now able to use SD OCT in daily practice as a simple and rapid way of performing OCT angiography too. Yet, as Philip Rosenfeld, Chairman of the Advanced Retina Imaging (A R I) Network explains, researchers at the forefront of retinal disease research always need “better, wider, deeper and faster imaging of the retina and the choroid.” These needs are critical – after all, patients’ vision is at stake. But they’re demanding too. Providing researchers with the best diagnostic instruments requires pushing the boundaries of not just medicine, but also optics, electronics, physics, mathematics and computer science. ZEISS does this in the knowledge that it helps further researchers’ discovery and understanding of diseases affecting the retina, and opens new frontiers of discovery in their quest for new clinical applications for different diseases. ZEISS’ approach to this is best described as “innovation through collaboration” and has undertaken a radical new initiative to supporting these top researchers: collaboration networks. Its first is the Advanced Retina Imaging (A R I) Network, a global consortium of the highest caliber of clinicians and scientists – those who are leading retinal research and other disciplines in ophthalmology, such as neurology and pediatrics. They, together with the engineers and scientists at ZEISS, are working to push the entire field of retinal imaging forward, and ultimately advance both clinical practice and patient care. What drives the A R I Network is the PLEX Elite 9000 from ZEISS. It is a SS-OCT instrument with a tunable laser centered at 1050 nm, a scan speed of 100,000 A-scans/sec at a tissue depth of 3.0 mm, and an axial resolution of 6.3 µm, with a 56° field of view… for the moment. Let’s revisit Philip Rosenfeld’s words, this is: “better, wider, deeper and faster imaging of the retina and the choroid.” This wide-field high-resolution visualization provided by the SS-OCT and OCT Angiography imaging modality of the PLEX Elite platform expands clinicians’ ability to examine the critical microstructures and microvasculature of the posterior segment at any depth of interest, from vitreous to sclera. ZEISS’ approach with the PLEX Elite system is much like that of a “Formula 1 concept car.” It’s not just an SS-OCT; ZEISS views it as “an open platform for innovation” that will regularly receive the latest technology – the best that ZEISS’ engineers and scientists can provide. Clinicians and researchers will make requests for new features or a different way of doing things – and ZEISS will respond by further developing its technology to meet those requests. The A R I Network members are then able to evaluate those advances and see if they truly make a difference in the clinic. Further, this rapid, iterative development, performed in collaboration with the A R I Network, will result in knowledge of what works, doesn’t work, and guide the future development of all OCT instruments – not just the advanced research models – meaning more patients will ultimately benefit. Importantly, the recent US FDA clearance will help US members of the A R I Network to more easily enroll patients and may facilitate faster Institutional Review Board (IRB) review for protocol approval of research further accelerating the pace of research. The A R I Network with the ZEISS PLEX Elite 9000 at its core supports researchers in the potential to discover and shape future clinical applications for ophthalmology and beyond to other disciplines in medicine – the potential is limitless. This new model of collaboration is the engine that will advance the standard of patient care in the future. The availability of ZEISS PLEX Elite 9000 in particular markets is dictated by the ARI Network steering committee and available regulatory pathways.
Medicine is becoming increasingly more personalized for the needs of each patient. As our understanding of genetics continues to grow, gene tests and therapies will become an ever-larger part of eyecare. And one company that’s bringing the personalized medicine revolution to ophthalmology is Avellino Labs. “Our Universal Test is the world’s first DNA test used to check LASIK candidates for genetic mutations that are associated with poor outcomes,” says Tara Moore, Avellino Labs Research & Development Director. Moore is also the Director of the Biomedical Research Institute at Ulster University in Northern Ireland, and there, she has amassed over twenty years of experience in progressing novel diagnoses and treatments for blinding eye diseases towards the market. “At Avellino Labs we have diagnosed over 700 cases worldwide of confirmed corneal dystrophy related to the TGFBI gene mutations. This contraindication for refractive surgery is easily detected using the non-invasive Avellino genetic test and I cannot emphasize strongly enough how important it is to eliminate any potential of such corneal dystrophies as part of the pre-screening process for refractive surgery,” adds Moore. Headquartered in California, with operations in Korea, Japan, China and the UK, Avellino Labs is currently expanding its repertoire to include a diagnostic test for keratoconus. In a recent study involving more than 200 keratoconus patients, Avellino Labs used state of the art next generation sequencing (NGS) to identify genetic risk factors in nine to 21 percent of patients tested in Korea. Based on these findings, the company intends to launch a test to screen for mutations in Korean, Japanese and Chinese populations in 2017. To pursue its goal of developing gene therapies and delivering personalized medicine, the company has also entered into a collaborative research agreement with Ulster University. Moore explains: “We hope to develop new technologies, and create a therapeutic platform that’s applicable to a wide range of inherited ophthalmic conditions. We’re investigating CRISPR gene editing as a means of managing and potentially curing corneal dystrophies and other inherited eye diseases” Named a 2015 Technology Pioneer by the World Economic Forum, based on its potential to impact global health, the company continues to expand its offerings in molecular diagnostics, including further NGS studies of inherited keratoconus.
References
- NGENUITY® 3D Visualization System Operator’s Manual (8065830016), 20 July 2016. C Eckardt, EB Paulo, “Heads-up surgery for vitreoretinal procedures: an experimental and clinical study”, Retina, 36, 137–147 (2016). PMID: 26200516.
