- As the number of people with presbyopia continues to increase, so too does the size of this potentially lucrative market
- Corneal inlays are a proven method of presbyopia correction, and a number are commercially available today
- All, however, use synthetic materials, which hold a small risk of biocompatibility issues
- To avoid those issues, allogenic inlays that utilize donor human corneal tissue, which would have otherwise been wasted, are currently under investigation
Refractive surgery is a mostly elective, and therefore relatively lucrative market. As such, there has been a big focus on technological innovation in the field, and the last decade has seen great advances made in laser refractive surgery, clear lens exchange, and phakic IOL design. Thanks to the demographic bulge that is the baby boomer generation, the customer base is only likely to increase, with presbyopes representing the largest potential market for refractive correction. It’s precisely for this indication that corneal inlays have been – and are being – developed.
The available options
Corneal inlays are devices that are implanted into the stroma to help correct refractive errors, and three are marketed today in Europe for presbyopia correction: AcuFocus’ Kamra (a small aperture opaque inlay); ReVision Optics’ RainDrop (a hydrogel inlay that acts by causing corneal steepening); and Presbia’s FlexiVue Microlens (a small, hydrophilic acrylic refractive inlay). Although each takes a different approach to presbyopia correction, they all carry a small risk of biocompatibility issues because they are made from synthetic materials. In order to minimize the risk of corneal melt, these inlays are designed to be small and thin. Following placement, they can be associated with visual side effects such as halo, glare, and a corneal haze that may require topical steroid therapy to resolve (1)(2)(3). There’s also the risk of epithelial ingrowth, where cells accidentally dragged into the stromal interface grow around the corneal implant, leading to opacification and impaired vision. Furthermore, synthetic devices cannot be used as onlays – which are placed near the surface of the eye directly under the epithelium – as corneal epithelial cells fail to grow over them. In a bid to avoid the risks associated with the use of synthetic implants, research is turning to allogenic inlays, which utilize human corneal tissue.Taking the natural route
Allogenic inlays are not a new idea. The legendary Spanish ophthalmologist José Barraquer first experimented with adding human tissue for keratophakia in 1949, and in the years since, there have been several attempts to use human tissue for refractive purposes. But impeded by lack of predictability in outcome and high costs, the idea never reached the clinic. So what’s changed? Advances in laser technology have meant that there are better and more accurate ways to sculpt the donor tissue, plus developments in tissue banking procedures has meant that the availability and viability of donor corneal tissue has improved immensely over the years. Right now, there are two main sources of human corneal tissue for allogenic inlays: the lenticules extracted during a small incision lenticule extraction (SMILE) procedure, and donor corneas from tissue banks.Post-surgical salvage
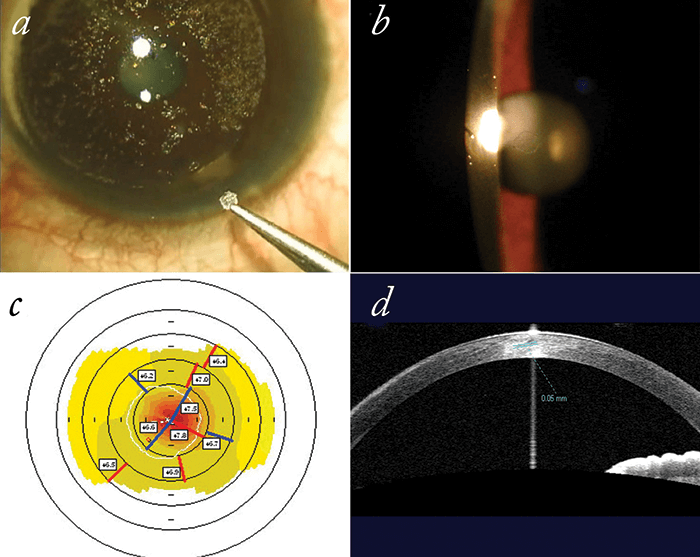
SMILE is becoming more widely adopted, and this means there’s an increasing amount of extracted lenticules that are available for salvage, modification, and placement in – or on – patients’ corneas. To date, there are several case studies reporting safe and effective outcomes of re-purposing SMILE lenticules for refractive purposes (4)(5). Recently, Soosan Jacob and her team from Dr Agarwal’s Eye Hospital in Chennai, India, have used SMILE lenticules as part of their PrEsbyopic, Allogenic, Refractive Lenticule (PEARL) corneal inlay procedure (Figure 1). “Presbyopia is the holy grail of ophthalmology and a very rapidly evolving field. This, together with limitations of currently available treatments, inspired us to start developing PEARL as a technique for presbyopia correction,” says Jacob. Their procedure involves cutting and fashioning myopic lenticules (of appropriate power and thickness) into shape, and implanting them in the non-dominant eye of presbyopic patients (6). “Prior to use, the SMILE lenticule is spread out and dried with a surgical sponge. We mark the anterior center with a fine, inked Sinskey hook, and use this to center the trephine when fashioning the lenticule,” describes Jacob. The lenticule is then implanted into a corneal pocket (created by programming the femtosecond flap to have a large hinge and a small side cut), spread out and aligned on the coaxially sighted light reflex (6). “The advantages of PEARL are that inlays made from human corneal tissue should automatically be biocompatible, and therefore be (in that respect) safer than the alternatives. The advent of femtosecond lasers has also made this technique possible – it gives us the ability to create custom tissue shapes with high precision,” explains Jacob. To date, 15 patients have undergone the PEARL inlay procedure. “The prolateness introduced by the inlay induces spherical aberration and provides increased depth of focus. We have been able to obtain very encouraging results, with improvements in near vision without significant deterioration in distance vision,” says Jacob, adding that “All patients reported vastly reduced dependence on spectacles.” Slit lamp examination showed the inlays to be centered and clear, and anterior segment OCT confirmed absence of haze, irregularities or perilenticular deposits. No interference with autoperimetry or fundal evaluations were observed, and minimal night vision symptoms were reported (6). “We have been pleasantly surprised by the ease of the procedure, how happy the patients are, and how they’ve managed to retain good visual acuity with good near and range of vision.” So what’s next? Jacob admits that, “As with any new procedure, challenges exist. So far, we’ve been working on further enhancing the shape of the lenticules to obtain the ideal refractive parameters. The challenges that remain are to make the procedure more widely available and accepted, as well as refining the procedure further.” To do this, the team have a larger study planned, and intend to collect longer-term follow-up data. They are also working to combine LASIK with PEARL for patients with refractive error. Jacob also noted that the “limited availability of suitable inlays might possibly be increased in the future through involvement of eye banks in tissue preparation. Cryopreservation of lenticules would allow transportation for reimplantation at other centers.”Donor-derived tissue
Another group using human tissue for refractive purposes are Allotex – a start-up company led by David Muller and Michael Mrochen – who are currently developing allogenic inlays and onlays sourced from donor corneas. “I had been following the development of various inlay companies, and whilst I noted the excitement for these products in the US, it was clear that biocompatibility was the major stumbling block for adoption outside the US,” says Muller. “The cornea does not react well to foreign bodies and using allograft material will always be the first choice.” Mrochen adds “Over the past years, clinicians have learned that the ‘cornea is not a piece of plastic’ and, therefore, we should not be surprised that it reacts if we implant plastic material.” Their idea is to make tissue available for the surgeon to customize prior to implantation. To generate the lenticules from donor tissue, the epithelial and endothelial cells are first “scrubbed off,” followed by e-beam sterilization. The tissue is then cut into multiple laminae of varying thicknesses (10–100 µm). The laminae are then cut into lenticules, and packaged ready for distribution (Figure 2). But aren’t donor corneas needed for more important things? Muller comments that that all tissue in their TransForm lenticules is “ethically sourced.” The company has an exclusive relationship with Lions Vision Gift in Portland, Oregon, which, according to Mrochen, “allow us to recover corneal tissue that has passed all ethical and health checks but is simply not suitable for corneal transplantation.” The lenticules can also be considered economically viable, as a single donor cornea yields multiple lenticules, and packaged lenticules are stable for two years. Prior to implantation, lenticules are customized by surgeons using their own laser system. Muller notes that “the lenticules will have the full metrology on them, so the complete three-dimensional shape of that product will be known.” Once re-shaped, the lenticule is then centered on the cornea and implanted. Muller explains, “As we are not limited to using only small diameter, very thin inlays, this will allow us to treat hyperopia as well as presbyopia, or any custom reactive change desired on the anterior surface,” adding, “It will also allow us to do onlays, placed just under the epithelium, that can be used for presbyopia, hyperopia and myopia.” They expect to implant their first inlays over the next 6–9 months. “We have relied on the observations of others implanting the SMILE buttons which show that human allografts are a viable refractive modality,” says Muller. “Onlays have been in use for more than three decades, and we know today that such procedures have a long lasting effect and are safe because of the biocompatibility,” adds Mrochen. Focusing on establishing the manufacturing processes and developing a “desktop” excimer system for lenticule customization, Mrochen comments, “it has become an exciting journey learning more and more every day about the new tissue addition technologies that can now be used in patients.”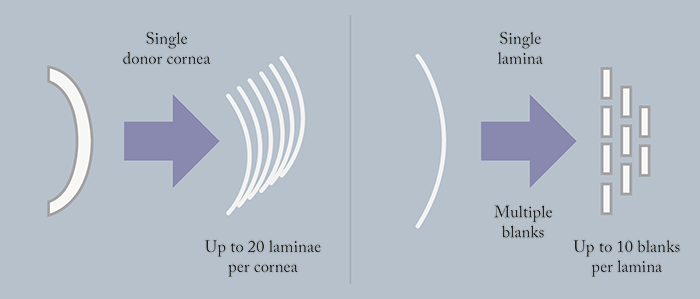
Looking to the future
So it seems that since the conception of the procedure over 60 years ago, things are changing, and the therapeutic use of allogenic inlays might be one step closer to reality. Although there is still some way to go in terms of optimizing procedures, in the years to come, the use of allogenic tissue to correct presbyopia – and other refractive errors – may become an important part of many refractive surgery practices. Soosan Jacob is an ophthalmologist at Dr Agarwal's Eye Hospital in Chennai, India. David Muller is the Founder and CEO of Allotex. Michael Mrochen is the President and COO of Allotex.References
- JL Alió et al., “Intracorneal inlay complicated by intrastromal epithelial opacification”, Arch Ophthalmol, 122, 1441–1446 (2004). PMID: 15477454. MM Ismail, “Correction of hyperopia by intracorneal lenses: two-year follow-up”, J Cataract Refract Surg, 32, 1657–1660 (2006). PMID: 17010863. ME Mulet et al., “Hydrogel intracorneal inlays for the correction of hyperopia: outcomes and complications after 5 years of follow-up”, Ophthalmol, 116, 1455–1460 (2009). PMID: 19651310. KR Pradhan et al., “Femtosecond laser-assisted keyhole endokeratophakia: correction of hyperopia by implantation of an allogeneic lenticule obtained by SMILE from a myopic donor”, J Refract Surg, 29, 777–782 (2013). PMID: 24203809. L Sun et al., “The safety and predictability of implanting autologous lenticule obtained by SMILE for hyperopia”, J Refract Surg, 31, 374–379. PMID: 26046703. S Jacob, “PEARL (presbyopic allogenic refractive lenticules – a new presbyopic corneal inlay”, (2016). Available at http://bit.ly/PEARLinlay. Accessed July 8, 2016.
