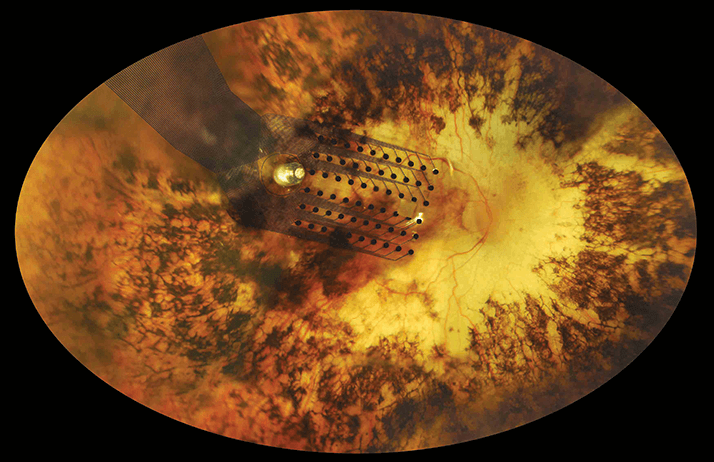
- Biomedical implants can partially restore vision to patients with retinitis pigmentosa
- The Argus II Retinal Prosthesis System is commercially available and nearly 100 patients have received the implant to date
- Real-life experience has shown that the implant not only improves visual acuity, but quality of life
- The economic arguments stack up: healthcare providers in the EU are beginning to fund the Argus II through various national reimbursement programs
Retinal degenerative diseases, like retinitis pigmentosa (RP) and macular degeneration, primarily affect photoreceptors, leaving the retina unable to sense light, and RP affects over 167,000 people in Europe today. In RP, however, some of the remaining retinal neurons – the bipolar and ganglion cells – retain their ability to signal and can be activated by well-established nerve stimulation techniques. This was the basic tenet of early electrophysiological studies that examined how electrical and magnetic fields could excite neurons in the visual system – phosphenes – to create the sensation of light. In the 1990s, clinical investigations that involved temporary implants of a stimulating electrode array in the eye of blind patients established that potentially useful spots of light could be created, even in a retina that had not been working properly following decades of disease and degeneration. Further, the use of multi-electrode array implants allowed people to perceive lines – and it was this discovery that ultimately led to the creation of implantable retinal prostheses.
Currently available prostheses
Today, a typical retinal prosthesis contains: an imager (such as a camera or photodiode array) that converts light to electrical signals; electronics that process the image and generate electrical stimulation; and an array of microelectrodes that stimulate the retina. Prostheses are typically categorized by the position of the array:- Epiretinal – on the top surface of the retina
- Subretinal – under the retina
- Suprachoroidal – between the sclera and the choroid.
Two retinal prostheses are currently available to patients: the epiretinal Argus II Retinal Prosthesis System (Second Sight Medical Products, Inc.) which is FDA-approved and CE-marked for the treatment of RP in the US and Europe, respectively; and the Alpha-IMS (Retina Implant AG, GmbH) which is a subretinal implant that’s CE-marked. To my knowledge, Alpha-IMS has not yet been commercially implanted. The Argus II is being routinely implanted in Germany, Italy and The Netherlands, and will soon be offered to patients in France. In June, the first Spanish patient was treated at the Barraquer Ophthalmology Centre of Barcelona. Argus II is also now commercially implanted in the US.
So far, so good
The scale of the improvements in Argus II patients’ vision is outlined in the latest data from a long-term, international study (NCT00407602) that followed 30 blind individuals (and when completed, reported on 26) with severe-to-profound RP over 60 months. The vision of most of the implanted people improved from a starting point barely detecting bright light, to locating objects and determining direction of movement. The best vision achieved so far is 20/1260 (0.02–1.8 logMAR). Users experienced improvements in performing their daily activities, including, for example: locating everyday items, identifying objects at various distances, crossing the road independently by following pedestrian crossings, and avoiding obstructions at head height while walking.Main findings of a 30-patient review
An international multicenter clinical trial was carried out to evaluate the safety and potential benefit of the Argus II in providing visual function to blind patients with severe-to-profound RP. As well as helping establish the safety and long-term reliability of the implant, the results also demonstrated quality of life improvements. • As judged by independent low vision rehabilitation experts, the Argus II System had, at some point during the study, a positive effect on the lives of 77 percent of patients by improving their functional vision and/or their well‐being.• Sixteen patients (62 percent) received the positive effects at the time of the study; the other four reported that the System had a positive effect earlier in the study. The System did not negatively affect any of the patients assessed.
• Eight patients (27 percent) demonstrated visual acuity improvement to >2.9 logMAR (best result at any follow-up visit).
• Trial patients consistently performed better with the Argus II System ON vs. OFF on orientation and mobility tests (e.g., finding a door and following a line).
European reimbursement
A recent, pan European study (6) concluded that Argus II is a cost-effective intervention for treating RP, with a low cost per QALY (Quality Adjusted Life Year) ratio, at approximately £11,700 per QALY. A number of EU countries’ state healthcare systems are, therefore, starting to offer reimbursement of the costs of implanting Argus II in patients with RP. In March 2014, the device was selected for fast-track funding under the French government’s ‘Forfait Innovation’ scheme, representing the first-ever medical device approved for support through this reimbursement program. The first treatment is scheduled for October 2014 and the French Ministry of Health believe that up to 36 blind patients with RP will have received the implant (7). In Germany, Argus II treatment is reimbursed under the annual NUB (Neue Untersuchungs- und Behandlungsmethoden) funding approval – a mechanism that facilitates prompt introduction of innovative healthcare products and devices. The reimbursement of the prosthesis is currently under review in England, where that country‘s NHS Specialised Commissioning is considering funding treatment for a limited number of patients with RP with profound vision loss. If the proposal is approved, the NHS would pay for a defined number of patients with RP and profound sight loss to receive the groundbreaking implant in a similar manner as in Germany and France.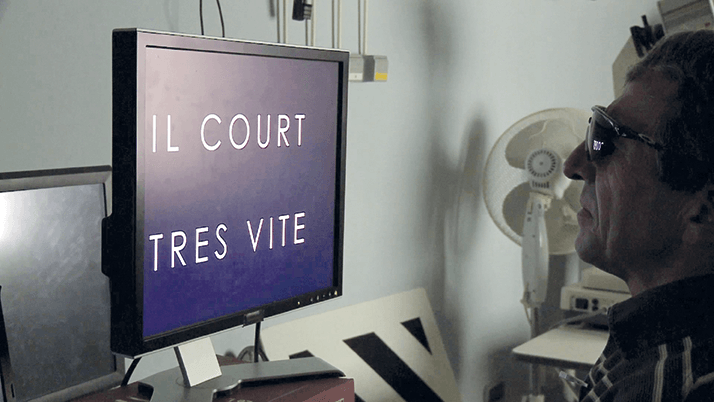
Making a difference today
The fact that the FDA has approved the device, nearly 100 patients have had the device successfully implanted to date, and the fact that national healthcare systems are beginning to reimburse the procedure represents good news for patients with RP – more will have a chance to have the implant, and see something of the world around them once again.
Burden of disease
There are estimated to be 167,000 people in Europe with RP causing an adverse impact on their quality of life and cost to society. How did we calculate this? Worldwide, an estimated 1.5 million people suffer from RP (1), which includes about 100,000 in the US (2). Pan-European data is not readily available, but we believe it is reasonable to estimate that the average prevalence throughout Europe is similar to the average prevalence within the US, and so the ratio of populations could be used to estimate the number of Europeans affected to be 167,000 in the 28 EU countries (3,4). Approximately one in four people with RP in the US has vision that is 20/200 or worse (legally blind) (5).Amanda Hayhurst’s background lies in healthcare journalism, having written for the UK’s Daily Mirror and the Evening Standard, and held senior editorial roles with both prominent consumer magazines and public relations companies – and currently runs one.
References
- R.G. Weleber, K. Gregory-Evans, “Retinitis Pigmentosa and allied disorders.” In S.J. Ryan (ed.), Retina. Mosby, St. Louis, pp 362–470 (2001). Foundation Fighting Blindness, “What is Retinitis Pigmentosa?” http://bit.ly/1w0e8rF, accessed August 27th, 2014. Eurostat, http://epp.eurostat.ec.europa.eu/, accessed August 27th, 2014. M. Haim, “Epidemiology of retinitis pigmentosa in Denmark”, Acta Ophthalmol. Scand., 233, 1–34 (2002). S. Grover, G.A. Fishman, R.J. Anderson, et al.,“Visual Acuity Impairment in Patients with Retinitis Pigmentosa at Age 45 Years or Older”, Ophthalmology, 106, 1780–1785 (1999). Forfait Innovation, “Marisol Touraine annonce le remboursement intégral de la prothèse épirétinienne Argus II”, http://bit.ly/forfaitinnov, Accessed August 27, 2014. A. Vaidya, E. Borgonovi, R.S. Taylor et al., “The cost-effectiveness of the Argus II retinal prosthesis in Retinitis Pigmentosa patients”, BMC Ophthalmol., 14, 49 (2014). doi:10.1186/1471-2415-14-49.
