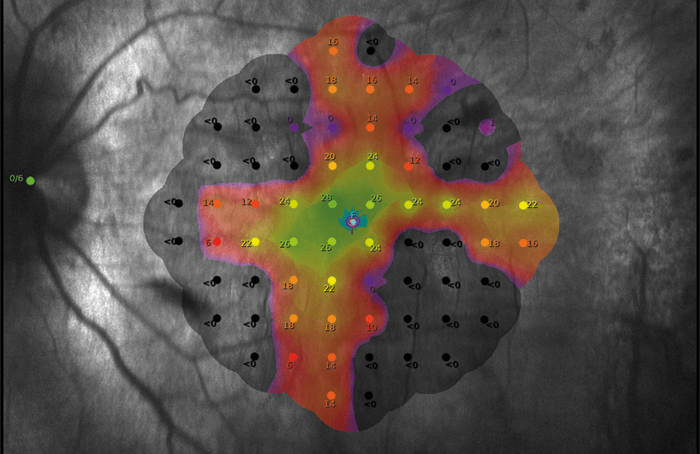
Two decades ago, Robert MacLaren began working on gene therapies. And the work is paying off, according to a recent publication describing vision improvement in patients who were given retinal gene therapy for choroideremia (1). Choroideremia is a rare (1 in 50,000–100,000) X-linked retinal degenerative disease. It manifests in childhood as an impairment of night vision, and is followed by peripheral vision loss, and then central vision loss later in life.
MacLaren led a 2011 clinical trial at the Oxford Eye Hospital, UK, which assessed subretinal injection of an adeno-associated viral vector that expresses Rab-escort protein 1 (REP1, the deficient protein in choroideremia). The paper in Nature Medicine notes that visual acuity improved in the 14 treated eyes over controls (median 4.5 letter gain, versus 1.5 letter loss, P = 0.04), with six treated eyes gaining more than one line of vision (>5 letters) – despite complications in two patients.
In a related press release (2), MacLaren said, “The early results of vision improvement we saw have been sustained for as long as we have been following up these patients and in several the gene therapy injection was over five years ago. The trial has made a big difference to their lives.”
Now, following the success of the Oxford trial, a pivotal Phase 3 trial in 100 patients is being conducted in nine countries across Europe and North America. The company leading it? Nightstar Therapeutics – a gene therapy spin-out founded by MacLaren and established by the University of Oxford and Syncona.
What chance of success? Well, the experimental gene therapy OTL-300 was given PRIME designation by the European Medicines Agency at the beginning of October, so the regulatory landscape for gene therapies is looking rosier than ever.
References
- K Xue et al., “Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia”, Nat Med, 24, 1507–1512 (2018). PMID: 30297895 University of Oxford, “Gene therapy breakthrough in treating rare form of blindness” (2018). Available at: http://www.ox.ac.uk/news/2018-10-09-gene-therapy-breakthrough-treating-rare-form-blindness Accessed October 19, 2018.
