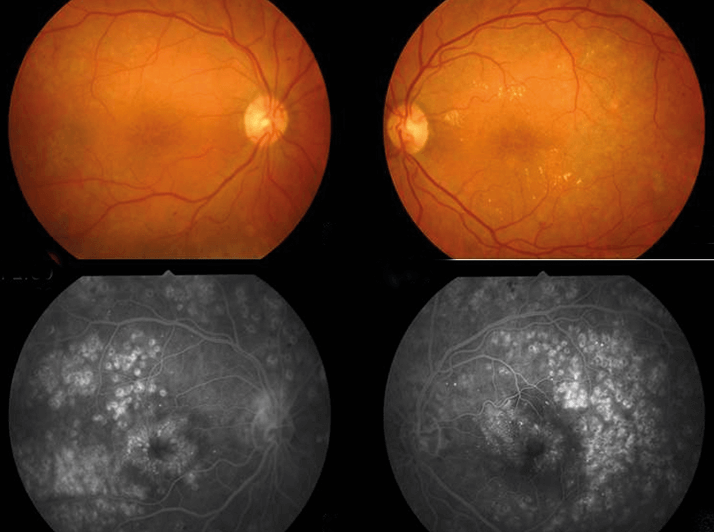
- DME results from the chronic inflammatory state caused by diabetes
- All current treatment modalities have their limitations: photocoagulation (poor efficacy), anti-VEGF (not all patients respond) and steroids (raised IOP and accelerated cataract)
- Slow-release corticosteroid formulations have led to a rethinking of their role in DME
- The benefit-risk balance may be in favor of steroid implants in those patients who are already pseudophakic
Most people with diabetes will develop diabetic retinopathy (DR). It’s an insidious disease; the early stages are silent, but it progresses relentlessly, and often a patient isn’t aware of vision problems until it’s fairly advanced. Diabetic macular edema (DME) is the single biggest cause of visual acuity reduction in people with diabetes. It can affect central vision from the early stages of retinopathy and is the most frequent complication of DR, particularly in older patients with type 2 diabetes.
The role of inflammation in the pathogenesis of DR is now well-established, and the repair processes have been associated with the universal endothelial dysfunction that occurs in diabetes. An emerging focus area of DR research is on the mechanistic link between the activation of subclinical inflammation and neurodegeneration: Müller cells show inflammation-linked responses when exposed to the diabetic milieu, and this environment can lead to activation of microglia and migration of macrophages, both of which may also play an active role in bringing even more inflammatory cytokines into the picture.
The disease processes in DME are mostly extracellular. Edema comes from the ancient Greek for swelling, οίδημα – in this case the swelling comes from the breakdown of the inner blood-retinal barrier, and the increase in tissue volume is due to an expansion of the retinal extracellular space, which is detectable with optical coherence tomography (OCT). When you have a situation of blood-retinal barrier breakdown, Ernest Starling’s observation that “edema occurs in a tissue when the rate of capillary filtration exceeds the rate of fluid removal from the perivascular interstitium” applies (1). With an open blood-retinal barrier, any change in the equilibrium between hydrostatic, oncotic and osmotic pressure gradients across the retinal vessels contribute to further water movements and may result in increased edema. Indeed, any increase in retinal thickness which is not attributable to a neoplasia comes from retinal edema.
DME therapy: now and then
For many years now, the standard of care for patients with DME had been laser photocoagulation. The procedure is not without its benefits – the principal one for most patients being an arrest in the decline in visual acuity loss (2). But only a minority of patients experience (slow) improvements in their edema and visual acuity, and after two years, one in five experience worsening vision once again (3). For more than a decade now, research has focused on the use of vascular endothelial growth factor (VEGF) antagonists. Intravitreal ranibizumab was approved by the European Medicines Agency (EMA) back in 2011 for DME based on the results of a number of clinical trials – REVEAL, READ-2, RESOLVE, and RESTORE (4–7). The US FDA followed suit, and gave its approval in 2012 based on the results of the Phase III RIDE and RISE studies. In all trials, ranibizumab use was associated with rapid edema reduction, significant visual acuity improvements and retinal thickness reductions, with the READ-2 and RESTORE studies demonstrating ranibizumab’s superiority over laser treatment. Very recently, the VEGF and placental growth factor inhibitor, aflibercept, received both FDA and EMA approval based on the Phase III VIVID-DME and VISTA-DME studies, respectively, that compared intravitreally-administered 2 mg doses of aflibercept every four weeks, with conventional macular laser photocoagulation – both studies demonstrated significantly improved visual acuity and significantly reduced central retinal thickness with aflibercept relative to laser photocoagulation (8).Although these two agents are currently unparalleled in terms of their safety and efficacy profile, not all DME patients respond and for some, efficacy fades over time. VEGF inhibition also fails to suppress any additional inflammatory mediators and permeability factors (other than VEGF) that are present in the diabetic retina: so patients with DME could benefit from a more comprehensive treatment strategy. This immediately provides a rationale for corticosteroid use. Corticosteroids act at both the biochemical and anatomical levels to exert their therapeutic actions; they reduce the expression of VEGF and other permeability factors in the eye, and they also suppress other inflammatory factors and the influx of leukocytes into the retina (9–11). Their use has, however, not been without risk. When it was introduced in the 1970s, many ophthalmologists embraced intravitreal injection of triamcinolone acetonide (IVTA) for the treatment of ME because it was effective in reducing macular thickness and improving visual acuity. But its effects were short-lived, which meant that patients needed frequent injections to maintain the effect (12). Furthermore, its use – as with all corticosteroids – was associated with cataract development and raised IOP.
Intravitreal implants
The short duration of action of IVTA (and other steroids) meant that research turned to developing sustained-release intravitreal implants that would release low doses of steroid over a long period – avoiding the requirement for frequent intravitreal injections and the wide intraocular drug concentration fluctuations that entails (13). Three such intravitreal implants have been developed; one that releases dexamethasone (Ozurdex, Allergan), and two that release fluocinolone acetonide (Iluvien, Alimera Sciences, and Retisert, Bausch + Lomb). Let’s look at each one in turn.Retisert
Retisert is sutured to the anterior eye wall and releases 0.59 µg of fluocinolone acetonide every day into the anterior part of the vitreous cavity – and is designed to do so for approximately two-and-a-half years. The implant has shown efficacy for the treatment of chronic non-infectious posterior uveitis (14). In DME, a four-year multicenter clinical trial found that Retisert significantly improved visual acuity and diabetic retinopathy severity scores (DRSS) relative to eyes that received standard of care (laser photocoagulation or observation) (15). However, this came at a cost; common adverse effects included cataract progression, elevated IOP and vitreous hemorrhage. Indeed, three years after implantation, 61.4 percent of implanted eyes had IOP elevation of more than 30 mmHg, compared with 5.8 percent of non-Retisert-implanted eyes, and glaucoma filtration surgery was required in 29.1 percent of implanted eyes. Retisert has not been approved for any indication by the EMA, and received its single FDA approval – for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye – back in 2005.Ozurdex
Ozurdex is comprised of 700 µm micronized dexamethasone, encapsulated in a biodegradable copolymer of lactic and glycolic acids, which slowly dissolves to release the steroid. The FDA had recently (June 2014) approved Ozurdex for the treatment of DME in patients who are “pseudophakic or are phakic and scheduled for cataract surgery”, but September 2014 saw the agency expand Ozurdex’s indication for the “general patient population being treated for DME.” This was also the same month that saw the EMA grant marketing authorization for the use of Ozurdex in “adult patients with visual impairment due to DME who are pseudophakic or who are considered insufficiently responsive to or unsuitable for non-corticosteroid therapy”, meaning that Ozurdex should also be available in European countries within the next few months. The evidence base for the regulatory authorities’ decision comes principally from a Phase II trial of the implant in patients (n=315) with persistent ME, secondary to various etiologies, including DME. Patients who received the Ozurdex implant went on to experience improvements in visual acuity, macular thickness and fluorescein leakage – and these benefits were sustained for up to six months (16). A further study that compared Ozurdex with observation in patients with persistent DME (of 90 days’ duration or more), showed that – compared with observation – Ozurdex was well-tolerated, and again produced significant improvements in visual acuity, central retinal thickness (CRT) and fluorescein leakage (17). Two randomized, sham-controlled Phase III trials of Ozurdex (containing either 0.35 or 0.7 mg of dexamethasone) were performed in patients (n=1,048) with DME, BCVA of 34–38 letters and CRT of ≥300 µm, followed for three years, with retreatment permitted at ≥6-monthly intervals. Pooled analysis showed both dexamethasone doses produced significant reductions in macular volume, CRT and disc areas of macular thickening on color photographs (all p<0.001 versus sham) (18).However, one non-randomized study has questioned Ozurdex’s claimed six-month duration of action (19). Fifteen patients with chronic DME (of over six months’ duration), unresponsive to bevacizumab, received the Ozurdex implant. Statistically significant improvements in central foveal thickness, relative to baseline values, were seen at one, two and three months post-implantation, but not beyond, and improvements in BCVA after implant placement were seen only in months one and two, waning afterwards. As the implant’s approved use in this indication is in its infancy, perhaps further study is needed to determine the actual duration of clinical benefit and the optimal time for retreatment in differing patient populations.
Iluvien
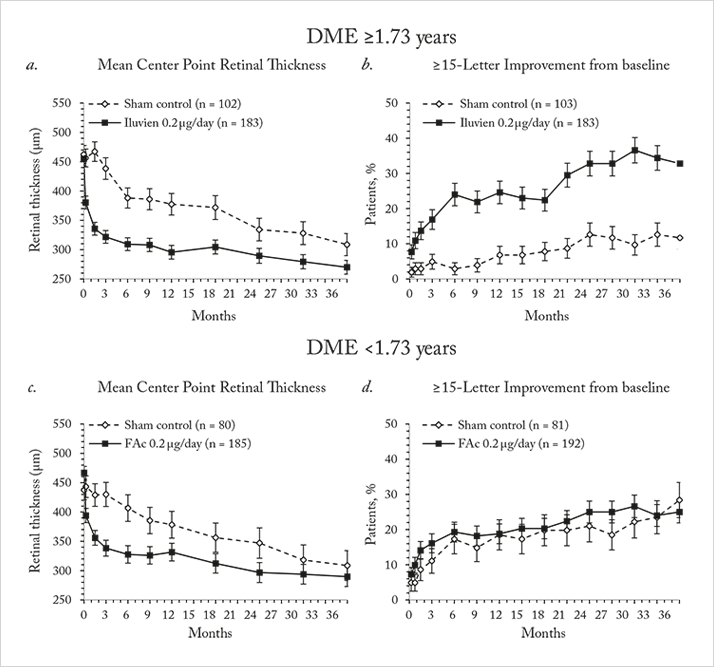
The Iluvien intravitreal implant is inserted into the vitreous cavity through a 25-gauge applicator in an outpatient setting, and contains 190 µg of fluocinolone acetonide, encased within an inert non-biodegradable cylindrical tube. Iluvien has very recently been approved by the FDA for use in patients with DME, adding to the EMA’s approval back in 2012 for its use in this indication. The approval wordings are different between the continents – the US label permits Iluvien use in patients with DME “who have been previously treated with a course of corticosteroids and did not have a clinically significant rise in IOP”, whereas the European approval is specifically for use in patients with DME that was “considered insufficiently responsive to available therapies.”The EMA and FDA approvals were based on the results from the Fluocinolone Acetonide for diabetic Macular Edema (FAME) study (20,21). FAME was comprised of two identically designed Phase III clinical trials that compared two doses of fluocinolone acetonide (0.2 mg/day and 0.5 mg/day) with sham injection over a three-year period – and all patients could receive standard-of-care laser photocoagulation six-weeks after photocoagulation. Both doses significantly improved visual acuity relative to sham, and patients in the treatment groups showed significant reductions in foveal thickness at all-time points over the follow-up period (Figure 1a). The majority (61.6 percent) of patients treated with the 0.2 µg/day implant did not require IOP-lowering medication and fewer than 5 percent required IOP-lowering surgery (Figure 1b, 2). The treatment efficacy of both fluocinolone acetonide doses were similar, but fewer adverse events occurred with the lower steroid dose, so the 0.2 μg/day implant was the one chosen to take to market.
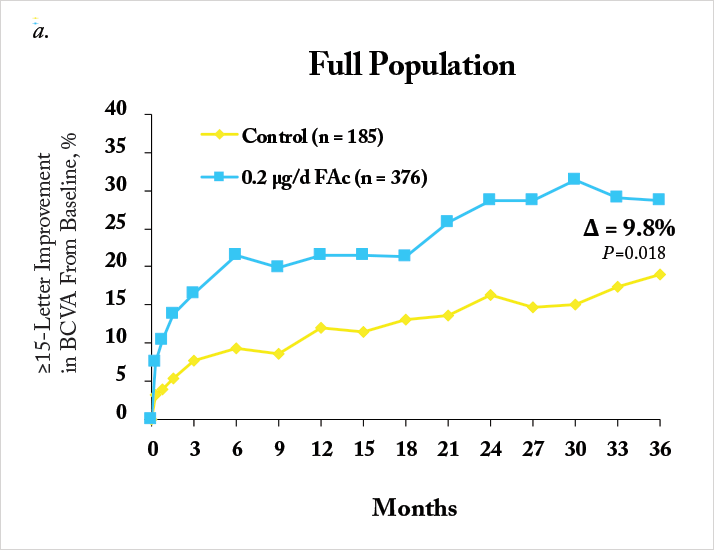
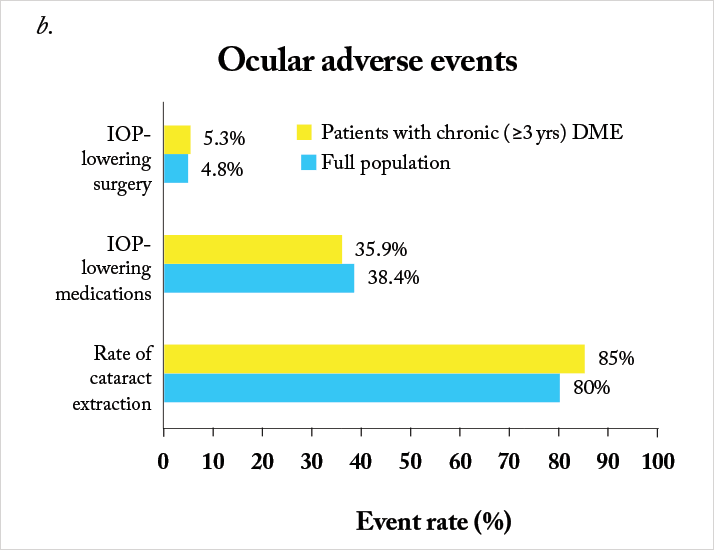
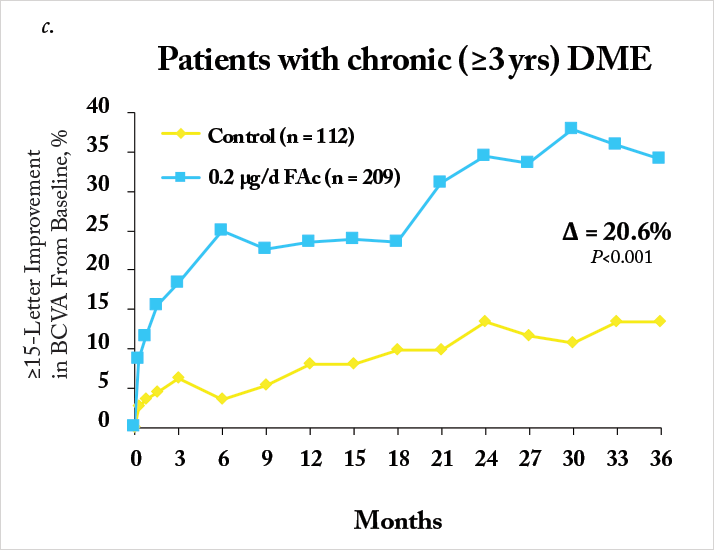
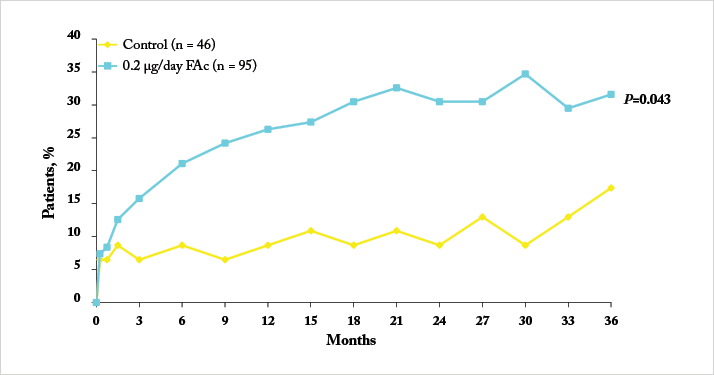
Notably, a significant number of patients enrolled into FAME had chronic DME (in this case, three years), as defined as the median duration of DME at trial enrolment. Iluvien’s effectiveness in treating DME was far more apparent in the chronic population compared with the overall population, with much greater improvements in both visual acuity Figure 1c) and foveal thickness being achieved (20,21). One issue, however, was the original calculation of median DME duration: it was particularly conservative in its approach. DME duration was calculated as: the year of study entry, minus the year of first diagnosis – plus one year (to ensure no patient with a diagnosis and enrolment in the same year had a DME duration of 0 years). A post-hoc analysis of the data that was based on the exact dates of diagnosis and study entry resulted in a median duration of 1.73 years. The FAME investigators reassessed the trial data using the 1.73-year duration of DME as the definition of “chronic” and compared it with the original study’s data (22). It turned out that 93 percent of patients that had DME defined as “chronic” by the old algorithm retained that definition with the new one, and that irrespective of how it was calculated, Iluvien’s efficacy was greater in patients with DME greater than the FAME trial’s median DME duration (Figure 3). These outcomes occurred despite a high incidence of cataract requiring surgery in Iluvien-receiving patients (81.7 percent after 36 months of follow-up), but even those who required cataract surgery experienced a mean increase in BCVA letter score of seven. A similar BCVA improvement was seen in patients who were pseudophakic at baseline (Figure 4).
Are we missing a trick?
Although at present intravitreal corticosteroid implant use is not recommended as first-line therapy, the impending requirement for cataract surgery in patients with DME may prompt us to reconsider this – if patients have failed to benefit from laser photocoagulation, then there is a solid rationale to consider steroid implants in these patients. The history of corticosteroids in DME is quite a long one, and interventions like IVTA have been associated with sufficiently high adverse event rates (principally accelerated cataract progression and IOP elevations) to warrant a pause before use. Intravitreal corticosteroid implants have surmounted or mitigated many of these issues, and it’s now possible to have an implant, that has a manageable safety profile, that exerts a therapeutic effect on DME for a prolonged period in a large proportion of patients. Considering the difficulties clinicians face in providing monthly anti-VEGF treatment, a single administration that lasts for a number of years is extremely attractive, particularly in pseudophakic eyes.José Cunha-Vaz is Professor of Ophthalmology at the University of Coimbra, Portugal and Editor-in-Chief of Ophthalmologica. He is also President of the Association for Innovation and Biomedical Research on Light and Image, a non-profit research organization dedicated to technology transfer.
References
- J.G. Cunha-Vaz, A. Travassos, 28, 485–492 (1984). Early Treatment Diabetic Retinopathy Study research group, Arch. Ophthalmol., 103, 1796–1806 (1985). Diabetic Retinopathy Clinical Research Network (DRCR), Ophthalmology, 115, 1447–1449 (2008). M. Ohji, T. Ishibashi, REVEAL study group, Invest. Ophthalmol. Vis. Sci., 53, ARVO E-abstract 4664 (2012). Q.D. Nguyen et al., Ophthalmology, 116, 2175–2181 (2009). P. Massin et al., Diabetes Care, 33, 2399–2405 (2010). P. Mitchell et al., Ophthalmology, 118, 615–625 (2011). J.F. Korobelnik et al., Ophthalmology (2014). Epub ahead of print. U.B. Kompella et al., Invest. Ophthalmol. Vis. Sci., 44, 1192–1201 (2003). H. Tamura et al., Invest. Ophthalmol. Vis. Sci., 46, 1440–1444 (2005). K. Wang et al., Biol. Pharm. Bull. 31, 1541–1546 (2008). S.G. Schwartz et al., Expert Opin. Pharmacother., 10, 1123–1131 (2009). S.S. Lee, M.R. Robinson, Ophthalmic Res., 41, 124–135 (2009). D.G. Callanan et al., Arch. Ophthalmol. 126, 1191–1201 (2008). P. Pearson et al., Invest. Ophthalmol. Vis. Sci. 47, 5442 (2006). B.D. Kuppermann et al., Arch. Ophthalmol., 125, 309–317 (2007). J.A. Haller et al., Arch. Ophthalmol., 128, 289–296 (2010). R.P. Danis et al, Invest. Ophthalmol. Vis. Sci., 55, ARVO E-abstract 5051 (2014). R. Lazic et al., Retina, 34, 719–724 (2014). P.A. Campochiaro et al., Ophthalmology, 118, 626–635 (2011). P.A. Campochiaro et al., Ophthalmology, 119, 2125–2132 (2012). J. Cunha-Vaz et al., Ophthalmology, 121, 1892–1903 (2014).
