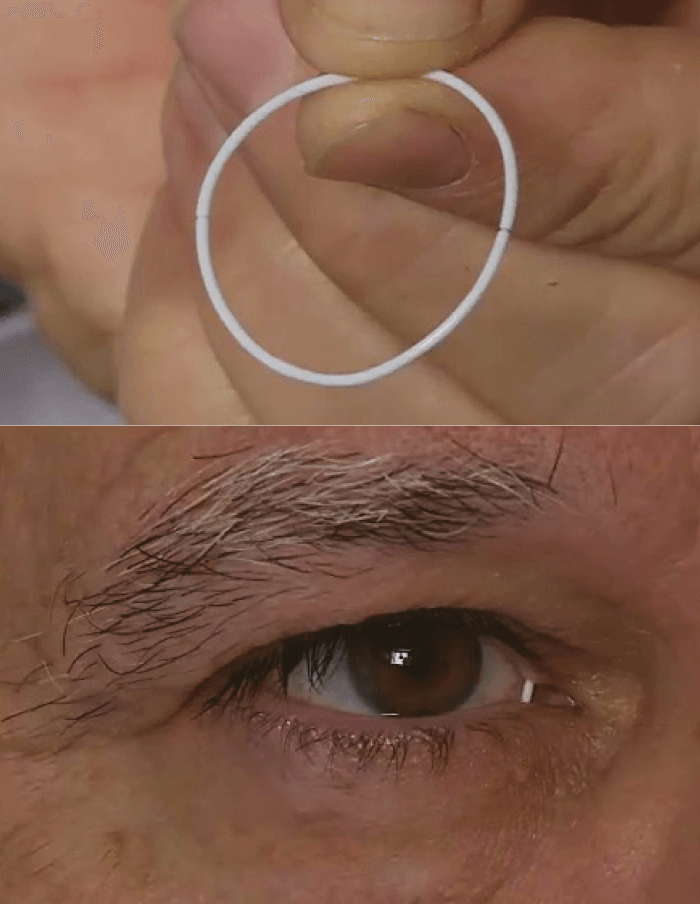
In patients with glaucoma, every missed dose of topical antiglaucoma therapy means poorer IOL control and faster vision loss. Regimen adherence varies from patient to patient, and missing a dose now and then isn’t the end of the world. But IOP control often requires multiple drops, and the effect of missing doses can quickly add up. Naturally, anything that can support medication compliance will help patients preserve their vision for longer. One approach that’s currently being investigated is a drug-eluting ocular insert ring (ForSight VISION5), which is placed on the upper and lower fornices of patients’ eyes (Figure 1). Comprised of a 1 mm bimatoprost-silicone matrix, the ring elutes therapeutic drug doses over a 6-month period. Results of a Phase II trial comparing the bimatoprost ring plus artificial tears with a placebo ring plus twice daily timolol 0.5% eyedrops in patients (n=130) with open-angle glaucoma or ocular hypertension controllable by monotherapy are now published (1). In this multicenter, randomized, double-masked trial, the primary efficacy measure was the difference in mean (diurnal) IOP change from baseline.
And the results? Over 6 months, reductions from baseline IOP ranged from 3.2 to -6.4 mmHg and -4.2 to -6.4 mmHg in the bimatoprost and timolol groups, respectively. The ring was well tolerated – at the end of the trial, 88.5 and 90.9 percent of patients still had the bimatoprost and placebo ring in place, respectively, and reported ocular discomfort rates were low for the bimatoprost (6.3 percent) and placebo rings (3.0 percent). However, of the three diurnal time points on each of the three days of IOP assessment (at weeks 2, 6 and 12), the bimatoprost implant was non-inferior to timolol at only two of them.This may have been because the trial was underpowered to detect the observed treatment effect, and this will be addressed in later Phase III studies.
Nevertheless, the bimatoprost ring was assessed under highly controlled clinical trial conditions where patients were compelled to be fully compliant with all dosing regimens. In the real world, the implant is being aimed at precisely those patients who struggle to do that. The ring has a large surface area – enough to carry a combination of ocular antihypertensive agents, and the fact that it can deliver drugs to the surface of the eye for an extended period with a high retention rate opens up the possibility that it might be used for the treatment of ocular surface disease, ocular allergy or post-surgical inflammation, all of which are under development.
References
- JD Brandt et al., “Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: results of a phase II randomized controlled study”, Ophthalmol, [E-pub ahead of print] (2016). PMID: 27157843.
