The eye enjoys a certain degree of immune privilege; the efficient blood-retina barrier, which prevents free movement of cells into and out of the eye, and ocular inhibition of immune-competent cells dampen local immune and inflammatory responses, and protect ocular functioning and vision (1). However, this ocular immune privilege is not fully understood, and thoughts are changing on the concept – with mounting evidence suggesting that inflammation may play a critical role in neovascular retinopathies (2)(3)(4). Jennifer Wilkinson-Berka of Monash University, Melbourne, wanted to find out more.
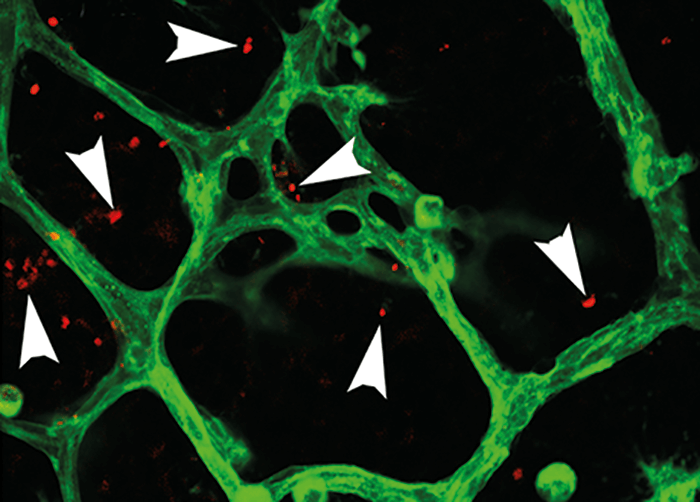
“I was interested in how inflammation contributes to the development of neovascular retinopathies, such as retinopathy of prematurity (ROP) and, in particular, if the adaptive immune system influences the innate immune system in the retina,” says Wilkinson-Berka. Using a characterized model of ROP in mice – oxygen-induced retinopathy (OIR) – the team set out to examine their hypothesis that Foxp3+ T regulatory cells (Tregs) might be recruited to the retina and that their abundance might decrease with the development of neovascularization (5). And the hypothesis rang true: Tregs were found to penetrate damaged retina in ROP (Figure 1). At postnatal day 13 (P13) – after neonatal mice were exposed to hyperoxia for 12 days – the numbers of Tregs was significantly higher in the inner retina compared with controls (p<0.001). By P18 – when there was extensive neovascularization and vascular leakage – the numbers of Tregs had reduced in the inner retina, and were visible within blood vessels. The team also found increased numbers of Tregs in lymphoid organs at P13 in OIR mice and saw a decrease in numbers at P18. Another key finding was that the team could modulate the Tregs – and help protect against disease. “We found that boosting the number of Tregs – and their immunosuppressive power – reduced severe vascular pathology in the retina,” says Wilkinson-Berka. Using a monoclonal antibody against IL-2 and adoptive transfer of Tregs to expand numbers of Tregs in the neonatal OIR mice, they found that retinal vaso-obliteration and neovascularization were reduced at both P12 and P18. Tregs were also shown to alter microglia activation, and in vitro studies confirmed that they reduced the numbers of pro-inflammatory mediators released by microglia.
“It was interesting to find that Tregs are naturally increased in the retina and lymphoid organs in response to acute tissue hypoxia in ROP, and then dramatically reduced when retinal neovascularization is established,” says Wilkinson-Berka. “It turned out to be a key piece of information for the treatment strategies we used, as it was important to prevent the decline in Treg numbers to reduce damage to the retinal microvasculature as well as the activation of retinal microglia.” Wilkinson-Berka hopes that their research will lead to an awareness of the contribution of the adaptive immune system to neovascular retinopathies. Could a new treatment approach be on the horizon? Wilkinson-Berka is hopeful: “Our next step is to develop immunotherapies that are safe, well-tolerated and effective in patients with neovascular retinopathies. My laboratory is very excited about the potential of our work to reduce the health burden of these sight-threatening retinal diseases.”
References
- R Zhou and RR Caspi. “Ocular immune privilege”, F1000 Biol Rep, 2, 3 (2010). PMID: 20348803. R Simo and c Hernandez. “Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence”, Prog Retin Eye Res, 48, 160–180 (2015). PMID: 25936649. P Sapieha et al., “Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life”, J Clin Invest, 120, 3022–3032 (2010). PMID: 20811158. A Agarwal et al., “Management of neovascular age-related macular degeneration: current state-of-the-art care for optimizing visual outcomes and therapies in development”, Clin Ophthalmol, 9, 1001–1015 (2015). PMID: 26089632. D Deliyanti et al., “Foxp3- Tregs are recruited to the retina to repair pathological angiogenesis”, Nat Commun, 8, 748 (2017). PMID: 28963474.
