Bionics is developing at an astonishing rate – and it seems almost inevitable that the technology will be increasingly commercialized. One company attempting to bring bionics – in this case, retinal implants – into the ophthalmic sphere is the Parisian-based “bioelectronics and brain machine interface technology company,” Pixium Vision. Leveraging its PRIMA Bionic Vision System, Pixium wants to partially restore central vision loss in patients affected by retinal diseases. The Ophthalmologist spoke with CEO Lloyd Diamond to learn more about the PRIMA System, clinical trial progress, and Pixium’s plans for the future.
Please introduce us to the PRIMA Bionic Vision System – and explain what it could mean for patients…
The PRIMA system aims to restore central vision by replacing the photoreceptor cells that no longer function due to age-related macular degeneration (AMD) and other retinal diseases. It does so by preserving the residual peripheral vision that advanced dry AMD or geographic atrophy (GA) patients have, while restoring the central vision that they’ve lost, allowing them to combine both.
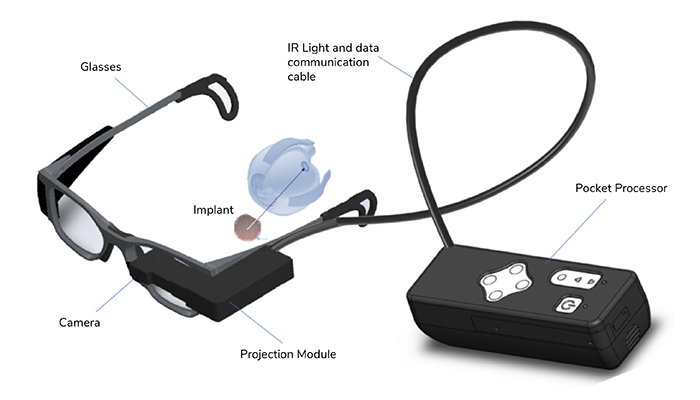
The neural implant itself is quite a marvel of modern technology. It was developed at Stanford University by Daniel Palanker, a well-known researcher in the area of tissue energy interaction. It’s a small microchip, thinner than a human hair, with 378 independent electrodes. These electrodes, along with the photovoltaic (converting light into electricity) properties of the implant, take on the role of a photoreceptor cell – they convert incoming light into an electrical signal. The device is implanted by a retinal surgeon in the subretinal space in a minimally invasive procedure that takes one-and-a-half to two hours.
The aim of this system is to give patients back quality of life and increase the level of independence. Since these patients have no central vision, they cannot do tasks that require the use of detailed vision. For geographic atrophy patients, for example, reading and facial recognition are two big concerns. If they like reading novels, with PRIMA they can do that again. If they take medication, they don’t need to have someone read them the prescription information. If they like to travel, PRIMA allows them to use public transportation again, without having to ask people for directions.
We are also developing improvements to our smart glasses, as well as co-development work with Stanford University on the next-generation implant. This next generation implant is up to 10,000 pixels – so an order of magnitude greater than what we currently offer. The goal with this technology is to give patients the ability to recognize faces at a distance and restore vision up to 20/20.
How does it differ from other retinal implant technology?
Pixium has demonstrated restoration of form vision, namely the ability to see objects based on light patterns projected onto the retina. This is very important because other technologies – including previous generations of retinal implants, be they cortical or epiretinal implants – only created phosphenes (the patient can see flashes of light). In fact, we are “restoring” some of the vision that patients have lost. We’re talking about a new generation of technology and the restoration of form vision – patients actually being able to see shapes and read. We have the first technology to facilitate that ability – and we’re very excited about that!
How far along are clinical trials with this technology?
We have three ongoing trials, and we’re in our final pivotal study – the PRIMAvera trial – in Europe. PRIMAvera will create the data that we’ll use for the CE mark. We also have a French feasibility study that is now more than four years post-implantation. And we have several publications in peer-reviewed journals on the safety and efficacy outcomes of that study, and we’ve shown at 48 months that patients had an average increase of 32 letters (1) – more than three lines on an ETDRS chart.
In the US, we also have an ongoing feasibility study where we’ve implanted patients; the 12-month data readout will be presented sometime early next year. In addition, the FDA granted us Breakthrough Device designation in March 2023, so we are currently negotiating our final investigational device exemption (IDE) study in the US with the FDA.
What are the challenges of developing this next-generation technology?
The challenge will lie in the ability to efficiently manufacture the implant. The current implant has 378 electrodes, and these electrodes are virtually flat on the implant. The next-generation implant will have up to 10,000 electrodes and the electrodes are three-dimensional. So, a challenge we face is working with our foundry partners to get our technology through the industrialization process, so that it can be reliably manufactured. We are reassured that we will be successful, as we have already demonstrated the ability to manufacture the current Prima neural implant at volume.
How do you see this technology developing in the future?
We understand what we need to do to maximize the effectiveness of the implant, so many of the future improvements will focus on wearables, namely the camera equipped glasses and the programmer that patients use to fine-tune their central vision, software which will likely one day make it to a smartphone. That means making the wearables more intuitive, incorporating the camera projection module into the glasses themselves, making them a lot easier for patients to use, and maybe more attractive to the outside world, more sleek. There’s also the potential to improve the utility of the device via software improvements and different modules, such as beaming Google Maps directly onto the implant or incorporating a gaming module that optimizes the types of games this patient population likes to play. Because diseases like GA, dry AMD, Stargardt’s, or retinitis pigmentosa can be isolating, creating a virtual community around these patients could be interesting.
References
- M Muqit et al., “Prosthetic Visual Acuity with the PRIMA System in Patients with Atrophic Age-related Macular Degeneration at 4 years follow-up,” medRxiv, [Online ahead of print] (2023). PMID: 38014146.
 Credit: Headshot supplied by Lloyd Diamond
Credit: Headshot supplied by Lloyd Diamond