- Neurotrophic keratopathy – the loss of corneal sensation – has a poor prognosis with limited available treatment options
- Current techniques for corneal neurotization can restore corneal sensation, but may be associated with significant morbidity
- I describe two minimally invasive techniques for corneal neurotization: using a cadaveric nerve graft and an endoscopic approach
- More ophthalmologists learning – and performing – these minimally invasive procedures may help more patients before devastating and irreversible damage occurs.
Neurotrophic keratopathy can be a devastating disease (See Box 1. Neurotrophic keratopathy pathology). Lost corneal sensation may lead to corneal scarring, ulceration and thinning, which can lead to corneal perforation and vision loss. It’s a debilitating condition for patients, and doctors have limited treatment options. Multiple ‘temporizing’ treatments try to decrease the chance of infection, ulceration and scarring, such as ocular lubricants, topical antibiotics, autologous serum drops, contact lenses, amniotic membrane grafts, and tarsorrhaphy. But none restore corneal sensation or the ability of the eye to respond appropriately to stimuli and maintain a healthy ocular surface. Furthermore, patients with neurotrophic keratopathy do poorly with corneal transplantation to replace the damaged tissue, because the same condition will recur in the corneal graft resulting in failure. The traditional standard of care for severe cases is to suture the eyelids together to protect the surface of the eye – a disfiguring procedure, which at best limits the patient’s field of vision and may lead to functional blindness if the entire palpebral aperture is closed. Some patients end up with permanently closed eyelids because corneal decompensation recurs upon re-opening. A novel surgical treatment has been described – corneal neurotization (1) – but the original techniques involve a significant undertaking. They involve either a coronal (ear-to-ear) incision with peeling the scalp and forehead tissues down to the level of the eye socket, or use of a nerve autograft harvested from a patient’s leg. Why? To route supraorbital and supratrochlear nerves from the contralateral side, tunnel them across the bridge of the nose, and to the corneoscleral limbus of the affected ‘anesthetic’ eye (1). These techniques for corneal neurotization have demonstrated successful outcomes – patients developed improved corneal sensibility, corneal health, and vision in some cases. But the means of getting there involve quite an invasive surgery with potential for significant donor site morbidity.
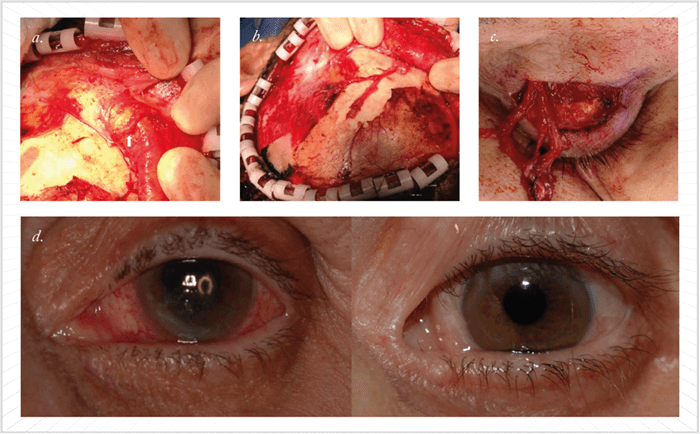
The sentinel patient with neurotrophic keratopathy who sparked my interest in this procedure presented to my clinic with severe corneal decompensation. In the four months following a retinal detachment repair surgery, she developed a non-healing corneal epithelial defect, thinning, and progressive loss of vision in her left eye despite maximal medical therapy. With hand motion vision, she was miserable and desperate. I wasn’t satisfied that there was nothing we could do besides suturing her eyelids together, and after extensive research to see if any other options were in existence, I stumbled across the paper by Terzis et al (1), which described the original corneal neurotization technique – four years after it was published. My cornea colleague and I decided to perform the procedure using a slightly modified technique – see Figure 1. The surgery took five hours and involved a large incision and extensive surgical manipulations. Fortunately, the patient ended up doing very well; her corneal sensation was restored with complete healing of her epithelial defect and improved visual acuity from hand motions to 20/30. I was able to open her eyelids, which were initially sutured together to protect her cornea (2). We were only the second center in the US to perform corneal neurotization; more groups have now reported performing similar procedures (3)(4)(5). It was a revolutionary concept for me because it worked so well, but I thought there had to be a better – and less-invasive – way. One group reported using a sural nerve autograft to restore corneal sensation through coaptation with the supraorbital or supratrochlear nerves (4)(5). Although less invasive than the procedure we and Terzis et al had performed, I still wanted to find an option that would avoid another donor site morbidity. My goal became creating a less invasive procedure that would still provide good results.
Tackling invasiveness
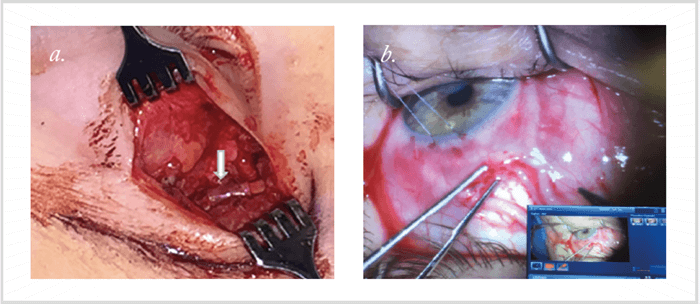
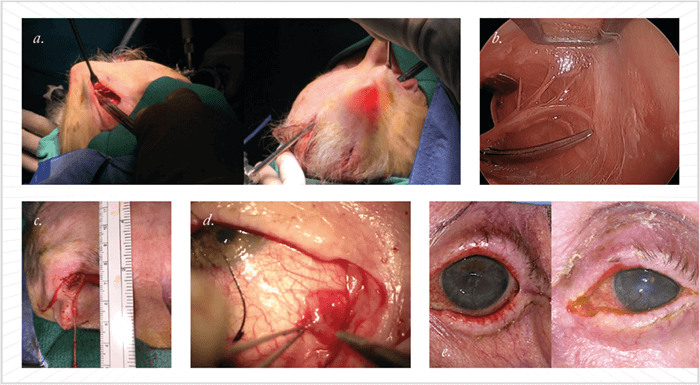
Surgeons use cadaveric nerve grafts to repair nerve injuries in other areas of the body, so I thought why not use them to restore corneal sensation? It would circumvent the need for more invasive surgery to harvest grafts from the patient. Using de-cellularized and processed cadaver nerves (AxoGen, Alachua, Florida), I’ve performed the corneal neurotization procedure in five patients (Figure 2). Within three months of surgery, most patients demonstrated restoration of corneal sensation and improved corneal healing. Some of the patients actually noted that they could feel eye drops in the operative eye for the first time since developing neurotrophic keratopathy. Using a Cochet-Bonnet esthesiometer we were able to track their progress objectively, and we’re thrilled with the outcomes – our results have been submitted for publication. We’re also starting a prospective study to examine and further understand the outcomes following procedure. We’ve also developed a second minimally-invasive technique – harvesting nerves using an endoscopic approach. This procedure involves making two small incisions behind the hairline – rather than a coronal incision – as well as a small eyelid crease incision (See Box 2. An endoscopic approach).First, we demonstrated the feasibility of using an endoscope for supraorbital nerve transfer to the corneoscleral limbus in two cadaver heads (6). We’ve also performed our endoscopic procedure in an 83-year old female patient with neurotrophic keratopathy from herpetic disease (7) (Figure 3). The affected eye had a visual acuity of hand motion at one foot, and a persistent corneal epithelial defect with dense corneal stromal scarring. Five weeks after the surgery, her epithelial defect had healed completely; and within three months of surgery we were able to demonstrate improvement in corneal sensation.- Neurotrophic keratopathy is a degenerative corneal disease characterized by decreased corneal sensibility and corneal healing.
- It has a prevalence of 1/2,000 – but it is likely under-diagnosed.
- It is a potentially blinding disease that can involve persistent epithelial defects, corneal scarring, neovascularization, corneal ulceration, perforation, or even loss of an eye.
- Clinical findings of neurotrophic keratopathy result from multiple mechanisms, including decreased sensory neuromediators, limbal stem cell compromise, epithelial compromise, decreased blink reflex and decreased reflex tearing.
- Etiologies of the disease include: infection (HSV, and so on), neoplastic and neurosurgical procedures, trauma (such as skull base fracture), ocular surgery, systemic disease (such as diabetes mellitus or multiple sclerosis), topical medication, chemical burns, congenital conditions, and advanced age.
- The most common cause is herpetic corneal infections; the second most common is intracranial pathology.
-
- Following induction of general anesthesia and injection of local tumescent anesthesia, make an upper eyelid crease incision in the donor upper eyelid. Dissect around 1 cm of the supraorbital nerve segment cephalad from the supraorbital foramen (or notch).
- Make two 1 cm vertical incisions just behind the hairline (5 mm posterior to the trichion). One incision should be placed in the midline and the other at a tangent to the contralateral medial limbus.
- Using a blunt endoscopic elevator, develop a subgaleal plane, stopping just cephalad to the previously isolated segment of supraorbital nerve.
- Using endoscopic guidance, dissect the rest of the supraorbital nerve cephalad through the scalp incisions, isolating terminal branches of the nerve.
- Tunnel the nerve branches through the upper eyelid incision.
- Make an upper lid crease incision on the side of the affected eye.
- Tunnel a curved hemostat from the eyelid incision on the affected side, in subgaleal plane, under the nasal bridge, and to the contralateral eyelid incision.
- Transfer the nerve branches from there to the upper eyelid crease incision on the affected side. After making a blepharotomy incision in the superior medial conjunctival fornix tunnel the nerve branches into the fornix of the affected eye.
- Place the nerve branches in the sub-conjunctival space through a conjunctival incision 8 mm above the 12 o’clock position, and secure the epineurium of the nerve to the sclera followed by conjunctival closure.
The merits of minimally invasive surgery
Both of our minimally invasive approaches have multiple advantages over the originally described corneal neurotization techniques. They’re less invasive – you’re not ‘scalping’ the patient or performing additional surgery elsewhere on the body to harvest a segment of nerve. Another benefit of a minimally invasive procedure is that it makes corneal neurotization more accessible to ophthalmic surgeons who may not be as comfortable with previously described approaches. If there is a procedure that more ophthalmologists can perform, then more patients will be likely to receive treatment. We’ll also be able to intervene earlier as patients will be more willing to undergo the procedure, rather than waiting until their disease becomes visually threatening. Currently, ophthalmologists who are aware of the procedure consider corneal neurotization a very morbid surgery. I am trying to change this perception – the minimally invasive procedures can be performed safely, with low complication rates, and are definitely within the scope of many ophthalmologists with proper training. The endoscopic approach is more technically challenging than grafting cadaveric nerves as it requires familiarity with using the endoscope, requires specialized equipment, and meticulous dissections in a small tunnel. Right now, corneal neurotization using cadaver nerve grafts is probably more reproducible, and I hope that in the future it might be picked up by more surgeons. I want to see corneal neurotization become a new paradigm in the management of neurotrophic keratopathy, and I want to see more patients cured from this disease. Ilya Leyngold is Assistant Professor of Ophthalmology at Duke University School of Medicine, Durham, North Carolina, USA. He specializes in cosmetic and reconstructive surgery of the eyelids, orbit, lacrimal system, and face.References
- JK Terzis et al., “Corneal neurotization: a novel solution to neurotrophic keratopathy”, Plast Reconstr Surg, 123, 112–120 (2009). PMID: 19116544. A Jacinto et al., “Ipsilateral supraorbital nerve transfer in a case of recalcitrant neurotrophic keratopathy with an intact ipsilateral frontal nerve: A novel surgical technique”, American Journal of Ophthalmology Case Reports, 4, 14–17 (2016). F Allevi et al., “Eyelid reanimation, neurotisation, and transplantation of the cornea in a patient with facial palsy”, BMJ Case Rep (2014). PMID: 25139921. U Elbaz et al., “Restoration of corneal sensation with regional nerve transfers and nerve grafts: a new approach to a difficult problem”, JAMA Ophthalmol, 132, 1289–1295 (2014). PMID: 25010775. RD Bains et al., “Corneal neurotization from the supratrochlear nerve with sural nerve grafts: a minimally invasive approach”, Plast Reconstr Surg, 135:397e–400e (2015). PMID: 25626824. M Tabor et al., “Endoscopic corneal neurotization: cadaver feasibility study”, Ophthal Plast Reconstr Surg, [Epub ahead of print], (2017). PMID: 28472009. I Leyngold et al., “Endoscopic corneal neurotization: technique and initial experience”, Ophthal Plast Reconstr Surg, 34, 82–85 (2018). PMID: 29194285.
