- MIGS represents a world of possibility in interventional glaucoma management because of their excellent safety and efficacy profile, as well as patient convenience
- To optimize surgical success, surgeons should assess several factors prior to attempting MIGS by conducting a thorough, pre-operative clinical examination
- While there are many approved and pending options available, a lack of data makes it difficult to come to definitive conclusions
- Glaucoma specialists and general ophthalmologists alike should collaborate to develop a framework that details when MIGS approaches are suitable – and for whom.
MIGS is a growing area of interest for glaucoma specialists and general ophthalmologists alike – and there are four reasons why. The first is the growing population and longevity of glaucoma patients; second, the financial burden, cost-ineffectiveness, and subsequent noncompliance to routinely prescribed/first line standard of care: pressure lowering eye drops; third, the reported toxicity and exposure to preservatives that these daily drops impose on the ocular surface; and fourth, the complications of filtering surgery, such as a trabeculectomy or tube shunts. But which MIGS procedure is right for your patient? To help you decide, here’s a concise overview of approved and emerging surgical interventions to decrease patient dependence on glaucomatous drops.
Surgical technique
Angle surgery frequently involves intraoperative use of the gonioprism. Manipulation of this device has a steep learning curve, involving coordination and maneuvering of the position of the patient, lens and microscope, while visualizing the tissues and structures of the angle. The Volk gonio lens (Volk, Tuscon, AZ) combines the prism with a fine-ring type stabilization system, which facilitates visualization without compression of the cornea. Prior to using the prism in conjunction with glaucoma surgery, surgeons should familiarize themselves with the lens intraoperatively alongside routine cataract cases, as adequate visualization seems to be the rate-limiting step in angle surgery.
Anatomic underpinnings
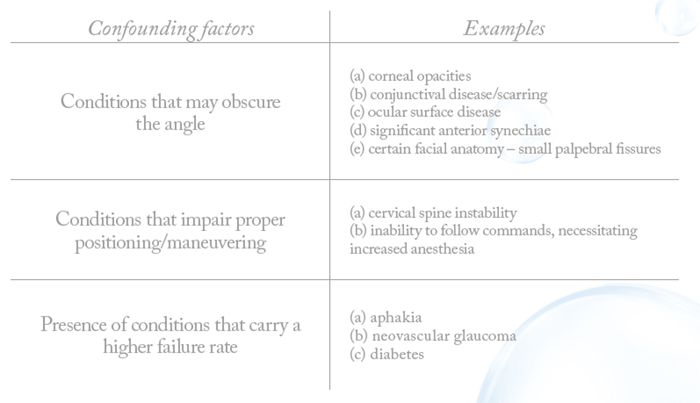
While visualizing the tissues and structures of the angle, it is important to orient with certain anatomical landmarks and know the normal/abnormal variants of each – and it is especially critical for eyes with preexisting pathologies, such as diabetic eyes, where the anatomy can be extremely delicate. If the first attempt at manipulating the tissues and placing the device or performing the procedure is unsuccessful, a second attempt in the same location is often impossible. A thorough, pre-operative clinical examination is vital to optimize surgical success (Table 1).
Getting on top of IOP
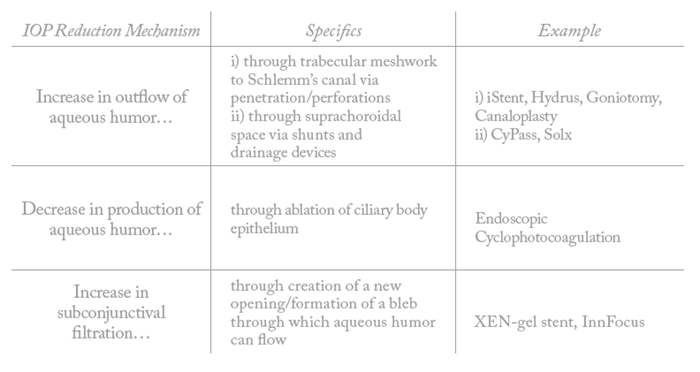
There are several different mechanisms by which each device/procedure exerts its desired effect on reduction of intraocular pressure (Table 2).
With respect to risks, the physician and the patient should be aware of the following:
- Schlemm’s Canal: this reservoir is similar to the physiological pathway of aqueous humor, and literature touts a higher safety profile. Reflux from collector channels can lead to hyphema. As with any glaucoma procedure, hypotony can occur. Devices like iStent and Hydrus can dislocate.
- Suprachoroidal space: this reservoir is dissimilar to physiological pathway, allowing for risks, such as cyclodialysis cleft with hypotony, late closure of the cleft with rapid rise in pressure due to atrophy of natural outflow structures, hemorrhage, inflammation, and hyphema.
- Sub-conjunctival space: similar to (2), this reservoir is not physiologic, and carries similar risks as above. Devices like the Xen or InnFocus can dislocate. In addition, though potentially as efficacious as trabeculectomies – a similar mechanism to filtration – it carries similar risks due to bleb-related complications, such as infection and fibrosis.
Approved MIGS
Xen (Allergan, Dublin, Ireland)
Placed through a clear corneal incision, the Xen device opens up a subconjunctival filtration pathway creating a fistula and resultant bleb. The bleb may cause conjunctival scarring, so an antimetabolite is often used. Formation of posterior blebs is preferable to anterior blebs, because of the decreased likelihood of bleb dysesthesia. This conjunctival procedure is relatively contraindicated in patients with aphakia, intraocular silicone oil or prior failed filtering/conjunctival surgery. Patients on multiple drops pre-operatively are typically told to substitute with oral acetazolamide at least one month prior, to optimize ocular surface. This device is only approved by the FDA in cases of refractory glaucoma unresponsive to drops and failure of initial surgery.
Limited studies exist that speak to the safety and efficacy of this device. In a clinical study of 30 eyes, mean IOP reduction was 6.2 mmHg at 12 month follow-up. Literature speculates that filtration bleb formation, as occurs in trabeculectomy, may result in complications such as encapsulation and subsequent scarring (1)(2)(3). In the study cited above, encapsulation of the filtration bleb was reported in one case (3.3 percent), which typically requires close postoperative follow-up with needling, revision procedures or prolonged course of topical steroids to reduce inflammation.
iStent (Glaukos, San Clemente, CA)
The iStent is a trabecular bypass device that is placed with a 25-gauge MST micro-canal, bypassing the trabecular meshwork. This device is ideally placed in the area of Schlemm’s canal with the highest density of collector channels, targeting drainage into large aqueous veins. Some investigators speculate the future use of imaging, such as optical coherence tomography (OCT), to accurately localize high-concentration areas of collector channels/aqueous veins pre- and perioperatively. The possible additive effect of inserting two or three stents instead of one is currently being investigated. The iStent inject, consisting of two stents placed at two different areas of Schlemm’s canal, is still in the process of FDA approval.
In one retrospective study of 134 eyes in 100 patients undergoing combined cataract extraction and implantation of iStent, mean IOP reduction was 3.6 mmHg at one year follow-up, with 94 percent of patients achieving their preoperative IOP goals (1)(4). Several head-to-head comparisons of iStent and phacoemulsification versus phacoemulsification alone have been shared in the literature. In a systematic review of 37 studies, iStent implantation was reported to have a 9 percent IOP reduction rate at 12 months follow-up, compared with 4 percent with phacoemulsification alone (5). The safety profile of iStent and cataract surgery was reported to be similar to phacoemulsification alone.
The most common complication reported with iStent implantation is transient hyphema (up to 19 percent). Other reported complications include stent obstruction/malposition (up to 10 percent), but reported cases typically did not require additional corrective intervention (4).
Goniotomy
A surgical procedure typically performed with a trabectome (NeoMedix, Tustin, CA), goniotome (Neomedix) or a Kahook Dual Blade (KDB; New World Medical), goniotomy removes a portion of the trabecular meshwork, increasing aqueous humor outflow. It does not penetrate the sclera, and is not associated with blebs. The most common reported complication is blood reflux; occasionally, cyclodialysis clefts with associated hypotony can occur.
Historically, this procedure was used mainly in the context of congenital glaucoma, where the procedure was incisional. There is a wealth of scientific studies that evaluate the efficacy of this procedure in the pediatric population, but more literature is needed to address its increasing use in the adult population. In the adult version, dual blade systems are used to excise a portion of trabecular meshwork. In a recent retrospective study of 71 adult eyes, there was a mean decrease of 4.6 mmHg IOP 6 months post-operatively (6).
ABiC
Ab interno canaloplasty (ABiC) uses a flexible microcatheter inserted through a clear corneal incision viscodilating and catheterizing Schlemm’s canal, the trabecular meshwork and the distal collector channels circumferentially. It is a modified version of the traditional canaloplasty, but does not require a suture to create tension and maintain aqueous outflow. An attractive feature of this procedure is that it restores the natural anatomical outflow system of the eye with no implantation of artificial devices. In a case series of 228 eyes reported by Ellex iScience, an IOP reduction of 8.1 mmHg was reported at 12 months follow-up. However, this procedure is technically complex and relatively long to perform. It can rupture Schlemm’s canal or travel into false channels with potential complications.
Cypass (Alcon, Fort Worth, TX)
Approved in the US in July 2016, Cypass has been used in Europe for over a decade. It creates a passage of flow from the anterior chamber to the suprachoroidal space. Supraciliary devices, such as Cypass, are associated with a higher degree of complications – such as hypotony and IOP elevation – than trabecular bypass devices, limiting their use to milder forms of glaucoma. In a study involving 167 eyes of 142 patients, mean IOP decreased by 4.3 mmHg at 12 months follow-up, with the most common complication being early hypotony (up to 23 percent) (4)(7). Reports of late closure of the cleft with rapid spikes in IOP are emerging.
Endoscopic Cyclophotocoagulation
Endoscopic cyclophotocoagulation uses laser technology to ablate the ciliary body epithelium to decrease aqueous body production. Its precise delivery of laser beams allows direct visualization of the ciliary processes without damage to the surrounding structures. In a randomized control trial of 636 patients, ECP and phacoemulsification was compared with phacoemulsification only; combined treatment resulted in an IOP reduction of 3.3 mmHg, with no significant reduction in IOP (8). As its efficacy has not been clearly demonstrated, it has not become popular.
MIGS pending approval
Solx (Solx Inc, Waltham, MA)
This gold microshunt, similar to CyPass, increases outflow from the anterior chamber into suprachoroidal collector channels. However, it does so ab externo, via trans-scleral dissection versus corneal incision. It is under FDA investigation undergoing phase III clinical trials, with preliminary clinical data showing about 9.3 mmHg mean IOP reduction at 12 months follow-up. Complications reported include anterior chamber inflammation and hypotony.
Hydrus (Ivantis, Irvine, CA)
Hydrus is an intracanalicular scaffold that is inserted into the nasal quadrant of Schlemm’s Canal, promoting flow directly into distal collector channels, bypassing the trabecular meshwork. It is currently in phase IV clinical trials for FDA approval, but has already been approved in Europe. In a randomized controlled trial of 100 patients, mean IOP reduction was 9.4 mmHg. Reported complications include transient IOP elevation (up to 20 percent), transient hyphema (up to 10 percent), and iris adhesion/synechiae (up to 20 percent) (1)(4)(9).
InnFocus (InnFocus Inc, Miami, FL)
Currently in the final phases of FDA approval, InnFocus is a small microscopic tube inserted through a surgically created scleral flap, shunting aqueous fluid from the anterior chamber to the subconjunctival space. It is implanted via conjunctival and Tenon dissection (ab externo), with formation of a diffuse bleb that extends posteriorly. Preliminary data discussed by several investigators show a mean IOP reduction of approximately 10 mmHg from preoperative baseline, half of which occurred in the initial postoperative period (3). There are risks of bleb-related infection, leaks, and fibrosis, and – as with any glaucoma surgery – hypotony is a potential complication.
Mighty MIGS?
The options for MIGS have increased exponentially over the years because of their numerous potential benefits over conservative treatment (eye drops) and more aggressive surgical options, such as trabeculectomy or tube shunts. MIGS procedures, along with advances in laser technology – for example, SLT, micropulse cyclophotocoagulation – could conceivably move to the forefront in glaucoma management because of their excellent safety and efficacy profile, as well as patient convenience. They provide a viable venue for earlier, long-term intervention for glaucoma with less need for strict patient adherence, and can be done concurrently with cataract surgery, reducing patient costs and operative/anesthesia risks.
There is no shortage of options in this growing sector of glaucoma treatment. Although comparisons between several MIGS devices and trabeculectomy do appear in the literature, more comparative head-to-head clinical studies between different MIGS devices need to be performed. Such comparison will better address the pros and cons of each option to better guide surgical management and patient selection. Though clinical trials are underway, the data points and investigation methods are not uniform, making definitive conclusions about the utility of each difficult. Standardized, universal evaluation of each of these devices and procedures needs to be devised and implemented, including the following criteria:
- Safety endpoint
- frequency of complications
- types of adverse events
- Efficacy end points
- complexity of surgical technique
- scope of tissue manipulation
- reduction in IOP
- reduction in medications
- Guidelines for patient eligibility and contraindications
- preoperative clinical examination
- severity and type of glaucoma
- number of preoperative glaucomatous drops
- prior failure of surgical/medical treatment
Glaucoma specialists and general ophthalmologists alike should collaborate to develop a framework that details when MIGS approaches are suitable – and for whom. Nevertheless, the growing myriad of options is promising; together, they represent a whole new world of possibility in interventional glaucoma.
References
- LE Pillunat et al., “Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents”, Clin Ophthalmol, 11, 1583-1600 (2017). PMID: 29440871. VT Perez-Torregrosa et al., “Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions”, Arch Soc Esp Oftalmol, 91, 415-421 (2016). PMID: 26995503. W Green et al., “Review of the Xen Gel Stent and InnFocus MicroShunt”, Curr Opin Ophthalmol, 29, 162-170 (2018). PMID: 29319544. DZ Chen et al., “Safety and Efficacy of Microinvasive Glaucoma Surgery”, J Ophthalmol. 2017, 3182935 (2017). PMID: 28512578. MS Malvankar-Mehta et al., “iStent with Phacoemulsification versus Phacoemulsification Alone for Patients with Glaucoma and Cataract: A Meta-Analysis”, PLoS One, 10, 0131770 (2015). PMID: 26147908. MD Greenwood et al., “Goniotomy with a single-use dual blade: Short-term results”, J Cataract Refract Surg, 43, 1197-1201 (2017). PMID: 28991617. H Hoeh et al., “Initial Clinical Experience with the CyPass Micro-Stent: Safety and Surgical Outcomes of a Novel Supraciliary Microstent”, J Glaucoma, 25, 106-112 (2016). PMID: 25304276. BA Francis et al., “Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma”, J Cataract Refract Surg, 40, 1313-1321 (2014). PMID: 25088629. N Pfeiffer et al., “A Randomized Trial of a Schlemm’s Canal Microstent with Phacoemulsification for Reducing Intraocular Pressure in Open-Angle Glaucoma”, Ophthalmology, 122, 1283-1293 (2015). PMID: 25972254.
