
What was your role in LumiThera’s inception?
I have been active in the pharmaceutical and medical device field for over 25 years. A few years ago, I got involved in the development of a mechanism called photobiomodulation (PBM). The technology was first developed around 30 years ago for back pain, and reduction of swelling in arthritis. It was used in a variety of dermal and muscular/skeletal indications, such as joints injuries. When I came across this intriguing mechanism, which uses light therapy to stimulate blood flow and energy production in the cells, it was being developed for neurodegenerative conditions, such as stroke, Alzheimer’s and Parkinson’s diseases. At a laser event I attended, I was approached by two physicians, who expressed an interest in using the technology to help patients with degenerative eye diseases. The concept was really exciting, but I was too busy at the time to pursue it. Nevertheless, a year later I met one of the same physicians, and they had conducted a small trial in the meantime; using PBM on 11 patients with dry AMD, the study showed improvement in vision. As luck would have it, one of the ophthalmologists – Robert Dotson – moved from Tennessee to my stomping ground in Seattle and we decided to further develop the concept for ophthalmic disorders, including AMD and diabetic retinopathy. And that’s how LumiThera sprang into life. Of course, it’s taken the experience of a variety of people from across different fields, deeper knowledge of the underlying principles of PBM, as well as improvements in lasers and light emitting diodes to get the company to where it is now.
Why target degenerative eye disease?
We have realized that, all around the world, the number of patients suffering from these conditions is steadily increasing, and the costs of diagnosing and treating them are growing exponentially. It is now vitally important to develop innovative and cost-effective therapies that can help patients at early stages of the disease. Our global vision is to utilize PBM with existing, new and advanced imaging, diagnostic and biomarkers to change the way we approach eye disease. Let’s not wait until vision is lost before we act. If patients are treated early, progression of the disease can be slowed down, vision impairment reduced, and it might even be possible to restore some vision; early treatment could have a profound impact on the patients’ quality of life. In the case of dry AMD specifically, there aren’t many options available to patients, and the treatment burden is significant. We feel PBM has a great chance of slowing or preventing vision loss in a huge number of patients worldwide who – until now – did not have access to other treatment options. It is a huge undertaking, but we are confident we can achieve our goal.
Tell us about the Valeda Light Delivery System...
The name Valeda comes from Latin and means “strong and healthy.” We created a multi-wavelength device, which uses PBM to strengthen retinal tissue and make it healthier, defending against the onset and progression of dry AMD.
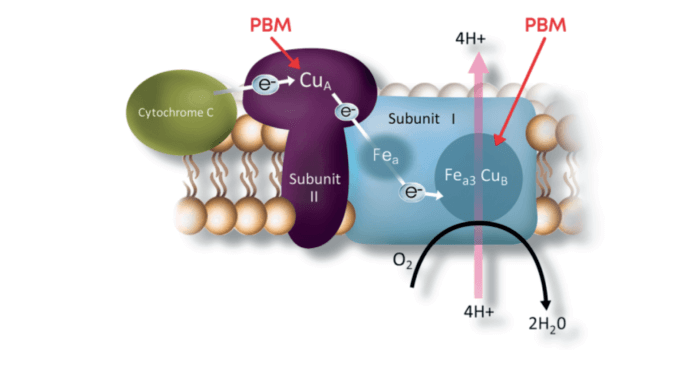
PBM works at a cellular level, activating mitochondrial respiratory chain components. It helps stabilize metabolic function and initiate a signaling cascade, promoting cellular proliferation and cytoprotection. Mitochondria use an important protein (Cytochrome C oxidase) for regulation – and the protein has been observed to be a key photoacceptor of light at two select wavelengths in the visible and NIR spectral range.
In PBM, photons are absorbed by photoacceptors in the targeted tissue, with secondary cellular effects, such as an increase in energy production and changes in signaling modalities. Activation of transcription factors leads to modulation in protein synthesis, proliferation, and improved cell survival for retinal cells, which are very energy-dependent. The Valeda Light Delivery System is the first approved treatment for dry age-related macular degeneration using photobiomodulation. Valeda is CE marked in the European Union.
The recently published study of PBM used as a treatment for dry AMD looked at 46 eyes of 30 patients, using the Valeda Light Delivery System. Patients underwent two series of treatments over the course of a year, three times a week for 3-4 weeks.
Best-corrected VA, contrast sensitivity, microperimetry, central drusen volume and drusen thickness, and quality of life were all measured, and PBM was found to have improved outcomes, with clearer benefits in patients at earlier stages of dry AMD. Visual acuity and contrast sensitivity significantly improved, and drusen volume and central drusen thickness diminished.
Can you share any details of clinical trials?
A prospective, double-masked clinical study in dry AMD subjects was recently published in Retina (see Box: LIGHTSITE I: Clinical Trials). Conducted in Canada, and partially funded by the National Institutes of Health (NIH) National Eye Institute (NEI), the study looked at patients with dry AMD and deteriorating vision, diagnosed with the disease at least seven years before the trial started. Patients received <5-minute PBM treatments per eye, which don’t require pupil dilation (so patients carry on with their normal activities after the treatment) and the treatments are actually quite relaxing. The study data demonstrated improvements in corrected visual acuity – up to 22 letters – as well as in contrast sensitivity. A full imaging analysis has been performed, and reductions in drusen were seen, which leads us to believe that cells are healthier following the treatment. We are conducting longer multi-center studies in Europe, and a companion study in the USA, and will have a lot more data by 2021.
What plans do you have for the future?
The platform LumiThera has created could potentially address a plethora of ocular complaints. It is being used in diabetic patients to treat open wounds, so possible options for this treatment include inflammation, and even acute trauma.
We are currently looking at the field of diabetic macular edema and diabetic retinopathy, to help support the growing number of diabetic eye disease sufferers around the world. Those patients have the option of intravitreal injections, but the associated costs and treatment burden of this invasive therapy are significant. The prospect of addressing the issues that both practitioners and patients face in this field makes our work very exciting.
Not for sale in the USA.
Visit www.lumithera.com for more information. MKT-0028 REV A.
References
- SN Markowitz et al., “A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration”, Retina, 9 (2019). PMID: 31404033.
