
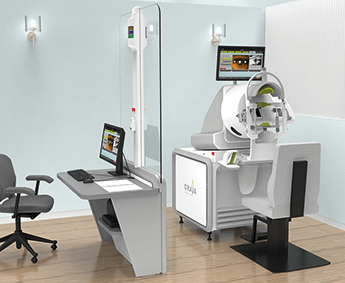
Oraya Therapy Availability Expands for Wet AMD Patients
The Oraya Therapy™ Stereotactic Radiotherapy for Wet AMD uses low-voltage, stereotactic, highly targeted X-rays to reduce anti-VEGF injections while maintaining vision.Oraya Therapy is a simple, non-invasive procedure, performed on an outpatient basis, and is intended as a one-time procedure. Nearly 200 patients have now been commercially treated with the Oraya Therapy, currently available in nine treatment centres across Germany, Switzerland, and the United Kingdom. The INTREPID study, initiated in April 2011, was a sham-controlled, double-masked trial to evaluate the effectiveness and safety of a one-time radiation therapy in conjunction with as-needed anti-VEGF injections for the treatment of wet AMD. The primary and secondary end points were met. The multi-national study included sites in Austria, Czech Republic, Germany, Italy and the United Kingdom and demonstrated reduced injections in a targeted patient population which obtained a 45% reduction in injections in two years. Full results of the 3-year safety evaluation from the INTREPID study were presented in September 2014 at the 14th EURETINA Congress, with physicians from three countries discussing their clinical experiences. The 2-year paper, published ahead of print in Ophthalmology, September 2014, is available on line. Oraya Therapeutics, Inc. develops innovative and non-invasive therapies for diseases of the eye. Founded in 2007, investors include Essex Woodlands Health Ventures, Domain Associates, and Scale Venture Partners. www.orayainc.com

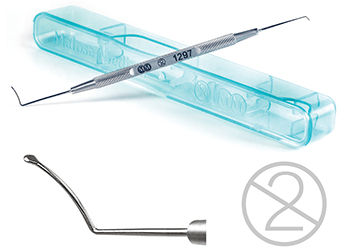
The Reinstein Lenticule Separator from Malosa Medical.
The first single-use instrument designed specifically for the pioneering SMILE procedureAdvances in femtosecond laser technology have led UK company, Malosa Medical, to develop a unique surgical instrument in collaboration with one of the world’s leading refractive surgeons. The instrument, which features both a pocketing hook and a unique separator tip, was developed alongside Professor Dan Reinstein specifically for the ReLEx® SMILE refractive procedure. The SMILE technique allows a flapless extraction of an intra-stromal lenticule through a single 2mm incision, minimising disruption to the biomechanics of the cornea and maintaining the structural integrity of the anterior stroma. The benefits of the Malosa tip design lie in the distribution of the vector forces in separating the stromal tissue. The thicker shaft and tip allow a more axial force and a higher force of separation while the expanded tip allows lenticule edge separation near the small 2mm incision without stressing or stretching the wound. The inclusion of a pocketing hook to open the access means that the entire procedure can be performed with a single instrument. Malosa Medical are specialist manufacturers of high quality single-use surgical instruments and procedure packs. With a wholly British-owned factory near Shanghai and UK- based packing and warehousing facility, Malosa deliver the best quality at factory direct prices. www.malosa.com/SMILE

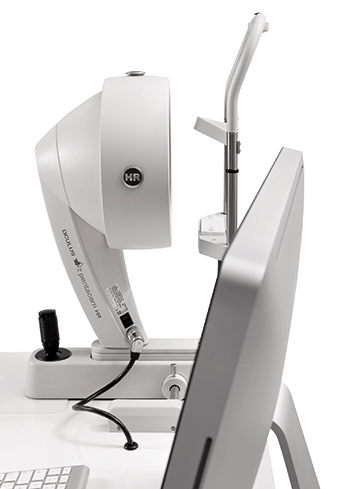
OCULUS Pentacam® - The Gold Standard in Anterior Segment Tomography
The Pentacam® offers a quick and comprehensive overview of the anterior segment and provides pachymetry, topography, height and curvature data for the front and back surfaces of the cornea.The ability to show and measure the entire anterior segment of the eye was a challenging concept. The result was presented at the AAO 2002. It was and still is a quantum leap for eye diagnosis: the OCULUS Pentacam®. The Pentacam® is an automatically rotating Scheimpflug camera without any Placido limitations. The unrevealed accuracy of the results as well as the unique and intuitive software tools made the Pentacam® technology the worldwide standard of care for cataract and refractive practices. Just to mention the unique Belin/Ambrósio software for early ectasia detection, the Indices Report with normative data, and the Cataract Pre-OP Display for customized premium IOL selection. Free software updates keep the Pentacam® always up to date – and there is more to come!
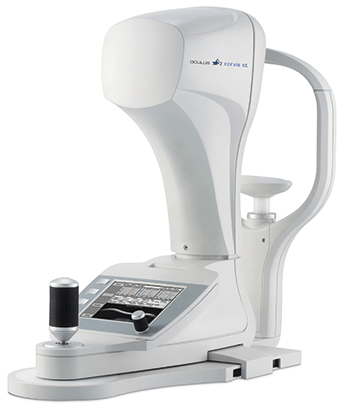
OCULUS Corvis® ST – the world’s first seeing tonometer
Corvis® ST records the reaction of the cornea to a defined air pulse using a newly developed high-speed Scheimpflug camera which takes over 4,300 images per second. First introduced in 2010, the OCULUS Corvis® ST shows pictures nobody has ever seen before: a high-speed Scheimpflug camera records the movement of the cornea!
These pictures open doors in a scientific and ophthalmological sense and open up possibilities in diagnosis of numerous diseases. Whereas classic tonometers merely calculate pressure values, the OCULUS Corvis® ST creates in only one second over 4,300 detailed ultra-high-speed Scheimpflug images of the deforming cornea. These videos provide ophthalmologists highly precise tonometric values along with a completely new view of corneal biomechanical properties.
OCULUS Optikgeräte GmbH is a family run business located in Germany since 1895. OCULUS consistently develops, produces and manufactures an extensive range of products at their headquarters in Wetzlar.
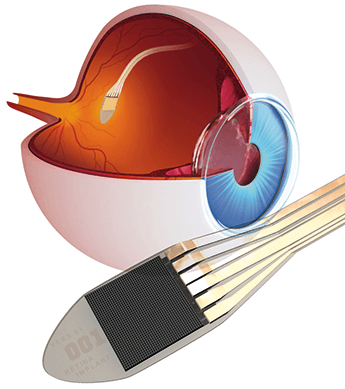
Retina Implant Alpha IMS restores sight to blind people
The Retina Implant Alpha IMS is a CE–certified subretinal implant, which is placed in the region of the macula lutea. In blind people, the function of destroyed photo-receptors is replaced by electronic light receptors.The Retina Implant Alpha IMS is a subfoveal implant, consisting of a small chip just 3mm² with 1,500 micro photo-diodes. Using this implant, it is arithmetically possible to regain a field of vision of 10°-12° and a decimal visual acuity of 0.04 (corresponding to a resolution of 25'). Incident light is captured dot by dot by photo-diodes and converted into electrical signals, so that at each point of the microchip a corresponding electrical charge is passed to the bipolar cells of the retina. In this way, the correct retinotopy of the connected bipolar cells is used to the full. The actual 70 μm thick microchip is placed subretinally under the macula lutea. This placement uses the natural microsaccades to refresh a static image. The angle of view of the eye is also used. Objects are recorded without any head movements. This principle has so far provided the best spatial resolution and visual acuity. Retina Implant AG is the leading developer of subretinal implants for blind patients. They began implanting human patients in 2005 and started a larger clinical trial in 2010. In 2013, their subretinal implant technology received CE mark. http://www.retina-implant.de/
CataRhex 3® - Phaco without limits
Weighing as little as 5 kg and fitting in any pilot case, the phaco machine can be mounted on any IV pole with a click – it is thus the epitome of portable equipment.CataRhex 3® offers the latest phaco technology, from clear lens exchange to the hardest lenses. The easyPhaco®Technology is available for 1.6, 2.2 and 2.8mm incisions with unparalleled efficiency and chamber stability. The newly developed CortexModeTM makes I/A with capsule cleaning noticeably safer and faster. Anterior vitrectomies can be precisely controlled with the high-precision flow control and the pneumatic Twinac cutting instrument and thanks to the integrated air pressure compressor they are available at all times. The HFDS® (High Frequency Deep Sclerotomy) ab interno function provides MIGS technology which elegantly enables combined glaucoma and phaco surgery. Efficiency and safety start with ease of operation. Displays and tactile keys are visible at a glance, all connections can be operated from the front, with the ergonomic multi-function pedal immediately responding to any foot movement. For over 50 years Oertli has been successfully developing, producing and selling surgical equipment that enables doctors and OR personnel to work in a safer, easier and more efficient way. The company is an independent owned family business and is headquartered in Berneck, Switzerland. www.oertli-catarhex3.com


Reducing refractive surprises during refractive cataract surgery?
Ignoring posterior corneal astigmatism during cataract surgery may lead to incorrect estimation of Total Corneal Astigmatism. Cassini Total Corneal Astigmatism (TCA) will help surgeons to understand the impact of posterior astigmatism on the magnitude and alignment of Toric IOL’s.Promoting Toric IOLs can be careful balancing act; on the one hand you want your patients to benefit from astigmatic correction in order to achieve optimal vision. At the same time you want to avoid refractive surprises and disappointed patients at all costs. With its unique and patented specular reflection technology, Cassini measures anterior and posterior astigmatism and provides you critical data to understand the role of posterior astigmatism for every patient individually. Cassini Total Corneal Astigmatism helps you to reduce refractive surprises and increases your confidence. So your patients can benefit from optimal visual outcomes. Cassini is an i-Optics product - i-Optics pioneers smart and superior eye diagnosis solutions that are affordable, fast and user-friendly for care providers worldwide to serve their patients best. http://i-optics.com/


1050 Hz Repetition Rate and 7D Eye Tracking
The SCHWIND AMARIS 1050RS is the new flagship of SCHWIND's excimer laser portfolio and strengthens the leading role played by AMARIS technology.This innovative laser system operates at an impressive repetition rate of 1050 Hz - currently the highest of all excimer lasers on the market - giving an extremely short ablation time of 1.3 seconds per dioptre. Another innovative feature of the AMARIS 1050RS is active 7D eye tracking in space and time. The Latency-Free Tracking considers the time factor, i.e. the seventh dimension. With this feature, eye movements occurring during the period between acquisition of the eyetracker image and triggering of the subsequent laser pulses are anticipated and pre-compensated. This results in zero latency for the laser system as a whole, which means even greater safety during laser treatment. The family company SCHWIND eye-tech-solutions develops, produces and markets a comprehensive product portfolio for the treatment of ametropia and corneal diseases. www.eye-tech-solutions.com


