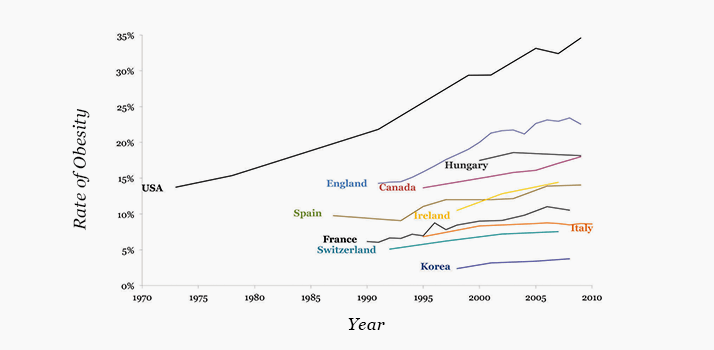
- Chronic systemic disease is increasing alarmingly, driven by skyrocketing obesity rates
- What is visible in the eye can predict vascular damage elsewhere
- Adaptive optics technology will soon be applicable to clinical practice, advancing retinal microvasculature imaging
- This will expand ophthalmologists’ front-line involvement in diabetes or cardiovascular disease
Chronic systemic diseases directly related to obesity, like heart disease, stroke and diabetes, already account for six out of every 10 deaths – and soak up a substantial proportion of all healthcare spending. Despite one major risk factor for cardiovascular disease – tobacco use – being curbed, the number diagnoses of diabetes and hypertension continues to rise inexorably. Skyrocketing rates of obesity, which the American Medical Association recently classified as a disease, mean that one ill has simply been replaced by another.
It is possible that ophthalmologists will play a leading role in the battle against chronic disease. Indeed, it could be argued that they already do. Eye specialists diagnose half of all type II diabetes cases, and cardiologists commonly refer many of their high-risk patients for fundus imaging. Yet, despite being widely commended for their role in saving vision, ophthalmologists’ contribution to the diagnosis and management of life-threatening systemic diseases is often underplayed. That may be about to change. The literature is full of descriptions (1) of how valuable current clinical imaging techniques are for generating information on the retinal vasculature. Fundus photography, scanning laser ophthalmoscopy (SLO) and optical coherence tomography (OCT) are all commonly used in the diagnostic chain, as well as in the monitoring of retinal and systemic manifestations of these chronic diseases. The utility of these techniques could be significantly increased if they were able to resolve the retina’s smallest vessels that are first affected by disease. Although fundus photography and SLO can reveal signs of microaneurysms in diabetes, SLO’s resolution is often insufficient to clearly distinguish them from other anatomical features present in healthy eyes. In arterial hypertension, these same techniques can reveal arteriolar narrowing and arterio-venous nicking – potential precursors to retinal vascular occlusion – but the same resolution limitations restrict what can be imaged to structures that might be considered macroscopic in relation to the retina’s overall size. With chronic cardiovascular disease, observing damage to the retina’s microvasculature will almost always mean that there is vascular damage elsewhere. In the case of hypertension, retinal microvascular damage can predict similar vascular damage in the brain, and in diabetes it may be a precursor to vascular damage in peripheral limbs. Because of this association, leading clinical researchers are actively pursuing ophthalmic imaging to diagnose and manage these diseases, as they discuss later in this article.
Systemic disease and the eye
Type II diabetes can go unnoticed for many years before clinical signs like lethargy, polydipsia and polyuria manifest themselves. Ocular symptoms of type II diabetes can often present first, meaning that patients are frequently initially diagnosed by an ophthalmologist. Presentation typically manifests as a loss in visual function due to proliferative diabetic retinopathy (PDR)but even when the disease is diagnosed in its earliest stages, almost all patients show signs of non-proliferative DR (NPDR). Although most cases of arterial hypertension are diagnosed during routine physical examinations, it is far from unheard of for it to be diagnosed by an ophthalmologist. Like diabetes, this occurs when the patient has already suffered acute loss in visual function. Hypertensive retinopathy (HR) is the second most common retinal vascular disease after diabetic retinopathy, and co-diagnoses of chronic hypertension with type II diabetes is common.There is no shortage of public awareness campaigns that target obesity, the leading underlying cause of the aforementioned diseases. Despite this, the rise in the numbers of patients becoming overweight and obese remains unchecked (see Figure 1). Strict adherence to dietary, exercise and pharmaceutical regimens is absolutely necessary to mitigate the effects of obesity on its associated chronic diseases, yet many patients are unwilling to do this, with dire consequences. Hypertension is the leading cause of death in the developed world, and diabetes is in the top five. Furthermore, diabetes is the leading cause of new blindness in people aged under 50 years. The impact of blindness on quality of life and productivity – for patients and their friends and families – is severe. The economic impact is equally substantial; for example, the total cost of obesity each year to US companies has been estimated at $13 billion. Patients with diabetes or arterial hypertension are encouraged to undergo comprehensive dilated ophthalmic examinations, the frequency of which depends on the severity of the disease. Putting new tools in the hands of ophthalmologists that will enable them to visualize retinal microstructural details will go a long way in helping doctors to save vision… and lives.
The adaptive optics era
First used in astronomical telescopes to great effect, adaptive optics (AO) technology awed the ophthalmic world in 1997 when Junzhong Liang, Donald Miller, and colleagues published the first in vivo images of human cone photoreceptor cells. AO works by measuring the effects of wavefront distortions – and compensating for them with a deformable mirror. In (astronomical) telescopes, this acts to remove the distorting effects of the atmosphere, increasing image resolution. With eyes, the wavefront distortions are caused by ocular aberrations in the many structures of the eye between the retina and the lipid layer of the tear film. Once corrected by AO, resolution is greatly improved (2).In 1997, AO technology had a very long way to go before it could become accessible for widespread clinical research and mainstream clinical applications. Prohibitive cost, instrument size and the complexity of operation were key factors that needed to be overcome. Recent developments have significantly reduced these barriers, but still, some ophthalmologists I have recently spoken to have voiced their skepticism about the value of visualizing individual cone photoreceptors. This is because foveal cones cannot be consistently imaged nor quantitatively analyzed in a reliable manner, and as the fovea is the primary region of interest for many ophthalmologists, their hesitation is understandable. Nevertheless, AO-enabled research devices have developed a solid track record within ophthalmology in terms of tracking the progression of multiple diseases that are associated with cone loss. Compared with current clinical imaging techniques, AO can track a geographic atrophy lesion over weeks, rather than over months, and AO can quantify cone depletion in retinitis pigmentosa and Stargardt disease. However, while being able to quantify cone numbers in cone-loss diseases using AO is an achievement, there is no effective treatment for these diseases. Consequently, the market for AO instruments – for this application – has been limited. Over the past several months, a number of publications and presentations (3–6) have focused on AO’s potential in non-invasive, high-resolution microvascular en face retinal imaging. Two experts, Marco Lombardo and Richard Rosen, offer their opinions on the following pages, describing their experiences to date and their views on how AO will impact the diagnosis and monitoring of systemic diseases. The groups of Lombardo and Rosen are just two of a number of groups worldwide investigating AO applications in ophthalmology. Michel Paques, of the Quinze-Vingts National Ophthalmic Center in Paris also has extensive experience in vascular imaging with AO. His group recently demonstrated that AO retinal artery imaging is not only feasible, it can produce consistent and reproducible results – in particular, to measure the thickness of the walls of arteries, an important parameter for patients with arterial hypertension. Already, Paques’ group has shown that parietal thickness increases linearly with blood pressure, and is currently evaluating longitudinal thickness changes in patients treated for hypertension. This will be of significant utility to ophthalmologists, cardiologists and neurologists in terms of tracking and mapping vascular disease progression, and will permit timely interventions that might prevent some of the worst consequences of cardiovascular disease – stroke, systemic embolism or myocardial infarction – from occurring. The knowledge that AO imaging in systemic vascular diseases is a viable and reproducible technique means that automated diagnostic software for longitudinal observation can be developed, which will be of utility to ophthalmologists and cardiologists alike for the tracking and mapping of vascular disease progression. Another prominent researcher, Stephen A. Burns at the University of Indiana, has published extensively on experiences with AOSLO. Establishing the biomarkers that will enable ophthalmologists to differentiate between healthy and pathologically-affected eyes is essential for the technology to find its way into everyday clinical practice. Burns’ group has generated data on the measurement and analysis of retinal blood flow velocity for this purpose. Much like Rosen’s AOSLO FA method, the combination of anatomical and functional (that is, physiological) imaging will greatly enhance the ability of eye specialists to diagnose diseases and to assess the efficacy of therapeutic interventions.
Where is it leading?
The fact that AO imaging technology has been adopted by many prominent research groups for vascular imaging in systemic disease may provide the proverbial “killer app” that Rosen mentioned. It comes down to basic economics.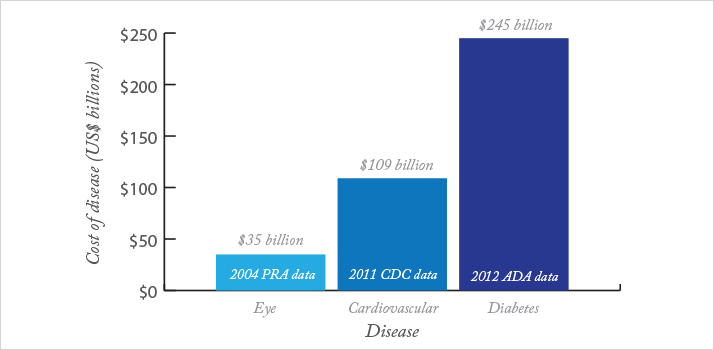
The total cost of the top four major vision disorders (age-related macular degeneration (AMD), diabetic retinopathy, cataract and glaucoma) in 2004 in the United States has been estimated by Prevent Blindness America to be $35.4 billion. That same report states that the numbers of patients with cataract and glaucoma far outweigh the numbers in the other two groups. Meanwhile, according to a paper published by the US Centers for Disease Control (CDC) in 2011, cardiovascular disease costs the US economy an estimated $108.9 billion annually. And the economic consequences of diabetes are more frightening still. In 2012, the American Diabetes Association estimated that diabetes cost the US economy $245 billion (see Figure 2). If an ophthalmologist can spot type II diabetes in the early stages – where it can be reversed through treatment and lifestyle interventions – the benefits to the patient and to society are potentially massive. Undetected, and left to develop, diabetes management requires expensive long-term therapy. Advancing visual and vascular problems result, ultimately, in patients who are highly visually impaired and immobile (some of whom will undergo limb amputation) that require an immense amount of medical care and familial support. It’s a similar situation for patients following myocardial infarction or stroke; both groups suffer from substantial morbidity and their care requirements are considerable. Already, ophthalmologists can, when needed, use anti-VEGF and photodynamic therapies to treat certain retinal manifestations of systemic diseases. In contrast, what cardiologists, endocrinologists, and general practitioners can provide for patients with chronic disease is surprisingly limited, mostly to prophylaxis. Ischemic strokes – particularly in patients with atrial fibrillation – can be prevented with oral anticoagulant therapy, which (despite the introduction of newer drugs that inhibit thrombin or Factor Xa) for most patients means taking a rat poison, warfarin. Cholesterol- and clot-filled cardiac arteries can have their filling – and eventual occlusion – slowed with statins and antiplatelet drugs, typically aspirin and clopidogrel. But these interventions only occur when patients are quite a way down the slippery slope of cardiovascular disease and diabetes is at an advanced stage. Diabetes drugs are moderately effective at controlling blood glucose levels, but many cause weight gain, and all have non-trivial event rates of particularly non-trivial side effects. Prevention is far, far better than the alternative; there are no cures. The implications are clear. Given that the first presentation of many systemic, vascular diseases is in the eye, ophthalmologists, using emerging imaging techniques like AO, offer a real chance of early intervention. They truly are at the front line of the battle, and have the ability – given a receptive patient – to not only save vision, but to save lives.
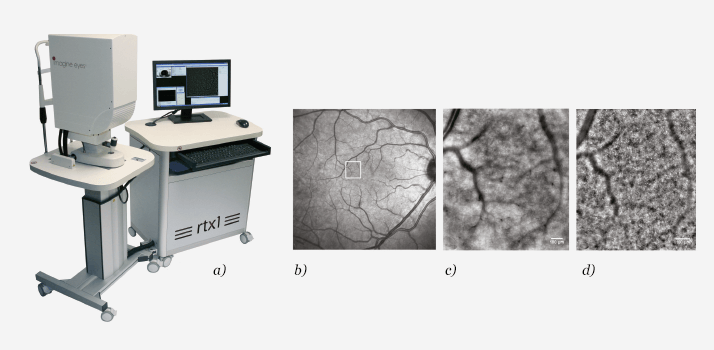
Adaptive optics imaging in non-prolific diabetic retinopathy
A conversation with Marco LombardoCan you describe your study?
We recruited eight patients with a diagnosis of type 1 diabetes and NPDR with no macular edema. Eight age-matched healthy subjects were recruited as controls. Patients and controls were submitted both to AO retinal imaging and conventional imaging using SLO and color fundus retinography (CFR). Using AO, the focal plane was adjusted to acquire images of the retinal capillaries of the inner nuclear layer in order to maximize the sharpness of vascular images. The capillary network of the inner nuclear layer was imaged 210-230 µm anteriorly to the photoreceptor layer. We used a semi-automated procedure to measure the retinal capillary lumen caliber in two regions of interest located close to the border of the foveal avascular zone (FAZ).What device did you use?
We used was an rtx1 AO fundus camera commercialized from Imagine Eyes (see Figure 3a). The device uses IR reflectance to provide 4° x 4° images at a resolution of 250 lppmm, which literature reports as ±2 µm in transverse resolution that can be acquired at different depths depending on the structures of interest.Did AO reveal information that the other techniques did not?
Retinal capillaries were not resolved by SLO or CFR in any eye. In AO images, the retinal capillaries appeared as faint vessel segments intersecting each other and forming a network of arterioles and venules both in NPDR and control eyes (see Figure 3b-d). The average lumen of retinal capillaries was statistically significantly narrower in NPDR eyes (6.27 ± 1.63 µm) than controls (7.31 ± 1.59 µm; (7)). On average, the retinal capillary lumen was 15 percent narrower in NPDR than in control eyes. Microaneurysms could equally be noninvasively observed in NPDR cases.How might this information be used in diabetes care?
The detection of pre-clinical abnormalities of retinal microcirculation may represent the real advantage of AO retinal imaging in the management of patients with diabetes. The capability to resolve retinal capillaries has been shown for all existing AO ophthalmic imaging modalities, namely AO-flood, AO-SLO and AO-OCT. Fluorescein angiography (FA) has been implemented in an AO-SLO, providing further in-depth investigation of the capillary network. The combined longitudinal assessment of the capillary density, capillary lumen caliber and the FAZ area by AO retinal imaging might provide valuable information on DR onset and progression. AO-SLO and AO-OCT can also characterize the blood flow in retinal capillaries, and a significant reduction in the capillary blood velocity in patients with diabetes has been shown as one of the earliest changes in DR. AO retinal imaging promises early detection of DR and monitoring of the progression of the disease with micrometric resolution. Moreover, AO can be used to evaluate the efficacy of new treatment options at a level that was heretofore unavailable.What needs to be accomplished for AO to become a part of everyday clinical practice?
Several factors must be resolved, including the development of easy-to-use software interfaces, fast image processing approaches and reliable analysis software. Improvements in AO-SLO have already enabled it to obtain images of retinal capillaries with incredible resolution – comparable to a histological section. While there are certainly challenges to the clinical application of AO retinal imaging, the rapid growth in the past few years suggests these will soon be overcome. A number of multi-disciplinary collaborations between clinical and non-clinical researchers have been initiated to resolve the specific needs of clinical AO imaging. Marco Lombardo is Senior Researcher at the Clinical Trial Research Center of the IRCCS Fondazione G.B. Bietti in Rome, Italy. He is responsible for the study protocol on AO imaging in patients and other projects related to the application of innovative biotechnologies to ophthalmology, including nanotechnology and regenerative medicine. These projects are a collaboration with CNR-IPCF, under the supervision of Giuseppe Lombardo.High-resolution AO-SLO fluorescein angiography
A conversation with Richard RosenWhat are the key differences between conventional FA and AO FA?
AO-SLO FA permits enhanced resolution, allowing finely detailed imaging of multiple layers of capillaries, microaneurysms, microvascular anomalies, capillary dropout and microleakage.What device do you use?
The AO-SLO used in our lab is a replica of the one developed by Dubra and Sulai at the Medical College of Wisconsin (see Figure 4a), with the visible channel modified for fluorescein angiography (FA) imaging (1). During AO-SLO imaging, simultaneous reflectance (790 nm) and fluorescence (488 nm) image sequences are acquired and registered.Does the system have any particular strengths?
While conventional FA can identify vessels based on their fluorescence alone, we are able to simultaneously image both the intraluminal space with AO-SLO FA (visible on the 488nm channel; see Figure 4b) and the vessel wall with AO-SLO reflectance (visible on the 790 nm channel). This allows us to delve into the delicate relationship between wall changes and luminal infiltrates. Differentiating between perfused and non-perfused vessels is also possible by comparison of AO-SLO FA images (functional perfusion map) and AOSLO reflectance images (structural).How did you work around any potential glitches?
The ability to gain early-phase information and monitor transit time of conventional FA is sacrificed in AO-SLO FA due to the more time-consuming technique of successive acquisition of individual small fields of 1.75°, which are then tiled together into larger montages offline. To accommodate the extended imaging sessions, oral fluorescein was chosen to provide a more consistent signal for a longer time than with an intravenous bolus. This is easier to administer and improves the safety and comfort of the procedure, when compared with intravenous administration.
How will your research improve diagnosis and management of vascular disease?
Improved resolution of fine capillary structures along with the ability to look at the photoreceptor mosaic and nerve fiber layer will lead to a better understanding of the anatomic basis of retinal disease. We hope that, with this advance in resolution to image the microvasculature, we will discover more accurate explanations for the pathogenesis of vasculopathy and visual malfunction. We hope this will lead to more targeted approaches to treatment and prevention of progressive deterioration. The ability to study microvascular disease in vivo will allow us to study microscopic changes dynamically over extended periods of time, giving an advantage over conventional FA and traditional histology. Following these changes in patients undergoing various treatment regimens will help us to better understand some of the underlying processes taking place within the macroscopic picture of disease progression and clinical response to specific therapies.We have already witnessed the impact of anti-VEGF therapy on capillary reperfusion and microaneurysm resolution and are in the process of studying the impact of laser photocoagulation and the fine structure of response of the retinal tissue. With this ability to study capillary dynamics clinically, we plan to investigate the changes that manifest as glaucoma, progressive vascular retinopathies, toxic maculopathies and episodic inflammatory diseases.Studying vascular remodeling after branch artery and vein occlusions may help us to better understand stroke recovery whereas studying the microvascular events which accompany capillary dropout and partial revascularization may help us understand similar processes in renal and cardiac disease. Understanding the various paths to progression of diabetic retinopathy may lead to more rational approaches to management.When will AO become part of everyday clinical practice, and what needs to be accomplished for that to happen?
AO will be ready for real-time once image processing improves, the speed of the study shortens the test to a length congruous with clinical encounters, and the cost and design of hardware allow the construction of a compact, semi-automatic instrument that can deliver an answer in a few minutes. Currently, the AO mirrors are hand-made and very expensive, which drives the price of the systems to levels that are not commercially sustainable. Due to current technical limitations of AO-SLO FA, we are only able to visualize 15° radius around fovea (1.75° at a time) whereas wide-field conventional FA can image as much as 180° in a single take. Clinical implementation will also demand an application, which depends on this level of imaging for monitoring, the so-called “killer app” – the way OCT is a surrogate VEGF meter and is critical for managing macular edema. Richard Rosen leads a team at the New York Eye and Ear Infirmary that includes Michael Dubow, Alexander Pinhas, Nishit Shah, Toco Chui, Alexander Gan, and Moataz Razeen. The group receives funding from Marrus Family Foundation, Bendheim-Lowenstein Family Foundation, Wise Family Foundation, Chairman’s Research Fund of the NYEEI and NIH.References
- R. Hazin, F. Lum, Y. J. Daoud, “Ophthalmic features of systemic diseases”, Ann. Med., 44 (3), 242-252 (2012). A. Dubra and Y. Sulai,“Reflective afocal broadband adaptive optics scanning ophthalmoscope”, Biomed. Opt. Express., 2 (6), 1757-1768 (2011). D. Rosenbaum et al., “Imagerie des artérioles rétiniennes par optique adaptative, faisabilité et reproductibilité”,. Ann. Cardiol. Angeiol. (Paris), 62 (3), 184-188 (2013). S. A. Burns, “Adaptive Optics Imaging for Studying Retinal Vasculature in Health and Disease”, Conference Paper, CLEO: Applications and Technology San Jose, California USA(2013). J Tam et al., “Subclinical Capillary Changes in Non-Proliferative Diabetic Retinopathy”, Optometry Vision Sci., 89, E692-E703 (2012). J. K. Sun et al., “Photoreceptor Mosaic Changes in Diabetic Eye Disease Assessed by Adaptive Optics Scanning Laser Ophthalmoscopy (AOSLO)”, Invest. Ophthalmol. Vis. Sci., 53, ARVO E-Abstract 4647 (2012). 7. M. Lombardo et al., “Analysis of retinal capillaries in patients with type 1 diabetes and nonproliferative diabetic retinopathy using adaptive optics imaging”, Retina, 33 (8), 1630-1639 (2013).
