Novaliq’s proprietary, water-free EyeSol® technology has been on the market in Europe, Australia and New Zealand -- first registered as ophthalmic medical devices in 2015. This year the company made significant progress with two accepted new drug applications which are currently in review by the U.S. Food and Drug Administration for the treatment of patients with dry eye disease. The company has bold plans to set a new gold standard in dry eye disease – but it doesn’t plan to stop there.
EyeSol® Technology
Novaliq’s EyeSol® technology opens completely new opportunities to cure, relieve, and prevent diseases in a range of therapeutic areas. The proprietary water-free technology uses ultrapure semifluorinated alkanes (SFAs) that are physically, chemically, and physiologically inert, thus exhibiting excellent biocompatibility and an excellent safety profile. Overcoming the limitations of water or oil-based topical therapies, EyeSol® technology therefore leads to higher bioavailability of active pharmaceutical products all as it stabilizes sensitive active substances, including proteins or peptides. The absence of preservatives, surfactants, oils, osmolarity, and a pH also leads to increased tolerability and higher rates of patient satisfaction.
Treating dry eye disease – taking two distinctive angles
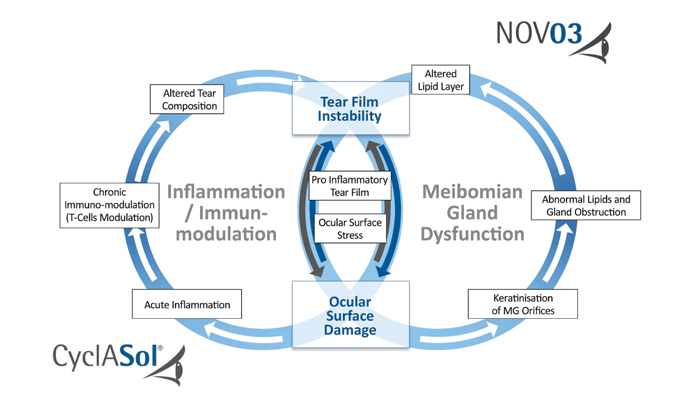
Distinct from all anti-inflammatory and immunomodulatory agents, NOV03 (perfluorohexyloctane) was specifically designed to treat the signs and symptoms of dry eye disease associated with Meibomian gland dysfunction (MGD). With its unique mode of action, NOV03 directly affects the lipid layer of the tear film preventing evaporation.
CyclASol, a cyclosporine ophthalmic solution, was designed to better address the chronic inflammation causing keratitis and the progressive corneal surface damage. This first-of-its-kind anti-inflammatory eye drop solution should bring a more favorable efficacy/tolerability profile and better bioavailability compared to other marketed therapies for dry eye disease; given that patients with moderate to severe DED associated with keratitis benefit most from the rapid onset of action, this differentiation will be pivotal.
If approved by the FDA, NOV03 and CyclASol® will all be game-changers in addressing important unmet needs in the treatment of dry eye disease that affects more than 16 million Americans (1).
Water-free therapies as a novel drug category
Beyond dry eye treatments, the EyeSol® technology will help develop entirely new product families across multiple therapeutic areas. The company has multiple exciting ongoing research programs beyond DED and is planning to use small and large molecules to make the next big step into topical therapies for the treatment of retinal diseases.
References
- American Optometric Association, “New study focuses on scope of dry eye disease in U.S” (2017). Available at: https://bit.ly/3UFUZwa.
