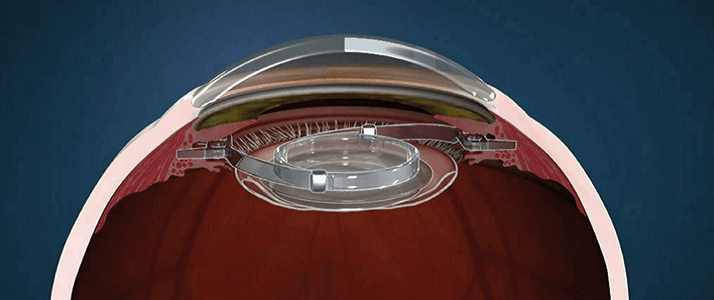
- Development tools used when creating a medical device have to be separately evaluated and validated by the FDA during the regulatory process, and approval of a device could stall if they are found wanting
- Now, a new program by the FDA aims to create a range of validated tools that will be made publicly available for companies to use when evaluating their products
- Progress has already been made by a taskforce involving members of the AAO, with a focus on accelerating the assessment of premium IOLs, and developing reliable methods of evaluating patient reported outcomes
- Ultimately, the program could result in a faster, simpler and more predictable pathway through US regulatory processes, helping to get more products onto the market
Launching a medical device in the USA can be quite a challenge. Generally speaking, it takes far longer to garner the approval of the US Food and Drugs Administration (FDA) for a product than it does to obtain a CE mark in the European Union – and meeting the FDA’s clinical data requirements can be a labyrinthine task. But a recent effort by the US regulator could help to simplify matters: the medical device development tool (MDDT) program is currently being piloted, with the goal of providing a battery of trusted tests for assessing medical devices, including IOLs – something that could considerably speed the regulatory approval process.
Removing redundancy
When developing an IOL – or any medical device for that matter – it’s important that the tools used to measure it are themselves validated. If the FDA determine that a tool used to assess your device is inadequate, or fails to adequately measure key performance parameters, your device will miss out on approval. But at the moment, despite the fact many manufacturers need to measure the same outcomes (or the same manufacturer wishes to measure the same outcomes in different devices), the FDA will evaluate the tool used to help develop the medical device independently every time. What this means is that in essence, companies are forced to have their tools repeatedly assessed every time they want to gain approval for a new market offering. But this is about to change. Draft guidance on the MDDT program, released late 2013, stated: “Previously, if there was interest in using a particular MDDT for multiple products or different clinical settings, each FDA device review team would typically evaluate the data justifying the MDDT use for each product or setting separately. Instead, if an MDDT is qualified […] the MDDT could be relied upon within the qualified context of use in the future, without redundant, detailed review of the suitability of the test” (1). This represents a huge change in the FDA’s attitude to MDDTs, and could potentially save both industry and regulators time, money and resources currently used on lengthy evaluations of the same tool in comparable contexts. It also has the potential to make device assessment faster, more efficient, and (crucially for developers of medical devices) more predictable too. It would benefit the regulators, as well, by removing the need to reconfirm validation each time a MDDT is used.Premium IOLs: The progress so far
For premium IOL evaluation, the FDA has joined forces with the American Academy of Ophthalmology (AAO) to work on several important aspects of the envisioned toolkit: safety and adverse events, objective and subjective assessments of accommodation, and subjective assessments of extended depths of focus. One set of measurements under development by the AAO taskforce has already been accepted onto the program – the tools for assessing patient reported outcomes (PRO).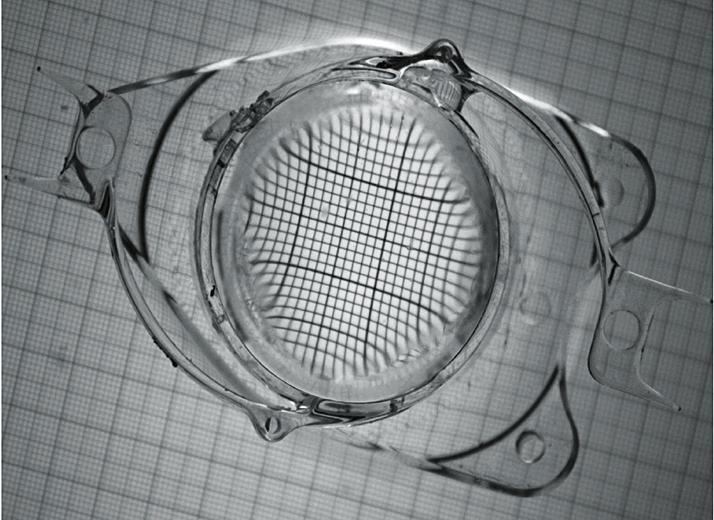
It is widely accepted that objective measures of IOL performance do not always correlate well with patient satisfaction. With this in mind, the taskforce has aimed to develop objective measurement tools such as questionnaires that include patient-focused definitions, and images of issues like dysphotopsias. Evaluations tailored for patients with higher visual function are also being developed, as are better measurements for assessing near work – an issue that has become increasingly important to patients. Work is currently underway at the Hoskins Centre of Ophthalmology in San Francisco to collate the expertise of industry, ophthalmologists and the FDA to continue work on creating a psychometrically-evaluated validated PRO questionnaire. A draft consensus statement will be released for comment, and published in Ophthalmology.
How can industry get involved?
The program is currently in its early stages, and the pilot has been launched to “pressure test” the process, in order to gather information for later changes to the program and MDDT development guidance (and also to qualify some initial tools). Participation is voluntary, but the FDA is currently accepting potential candidates that fit into one of three categories:- Clinical outcome assessments as a measure of treatment benefits
- Biomarker tests such as in vitro lab tests or medical imaging methods
- Nonclinical assessment models such as in vitro, animal or computer models (2,3)
The FDA has been working on MDDTs that could impact ophthalmology since March 2014, focusing on defining adverse events for premium IOL assessment, and recommending best practices in testing safety and objective accommodation. But there is a catch – if a MDDT qualifies, it must be made available to the public, and its use cannot be restricted to private entities. Propriety methods can be protected, but the tool must be available whether through sales, licensing or open sourcing. This could be a potential disincentive for companies, as developing an MDDT requires time and resources.
Accelerating approval
The MDDT program has the potential to speed up development and approval of medical devices, including premium IOLs, and get them to the US market faster. However, at this point it is unclear how enthusiastically industry will take up the challenge of developing products that will then be made available to the open market. But, the potential is there to develop a range of reliable tools which manufacturers can take advantage of to streamline development and assessment of their device, without needing to reinvent the wheel to do it.Glossary
MDDT: Medical Device Development Tool; a scientifically validated tool for assessing a clinical outcome, a test used to detect or measure a biomarker, or a nonclinical assessment method or model that aids development and regulatory evaluation of a medical device. Context of use: The use parameters for which an MDDT has been validated, defined in part by the product area, the stage of device development, and the specific role of the MDDT in development. Medical Device (as defined by the FDA): an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is: recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them, intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.References
- US Food and Drug Administration, “Medical device development tools – draft guidance for industry, tool developers, and food and drug administration staff”, (2013). Available at: http://1.usa.gov/1FRtrXN. Accessed September 18, 2015. US Food and Drug Administration, “Notice: Pilot program for qualification of medical device development tools”, (2014). Available at: http://1.usa.gov/1KvZMsy. Accessed September 18, 2015. M Eydelman, S Macrae, J Holladay, S Masket, “Regulatory Standards For Premium IOLs”, as presented at the Ophthalmology Futures European Forum, Barcelona, September 3, 2015.
