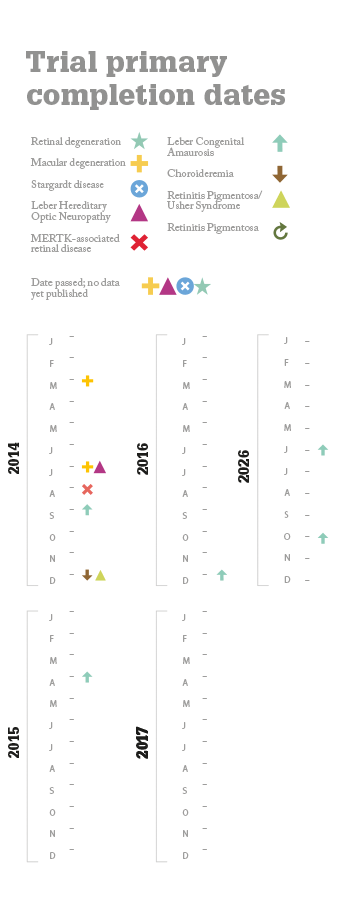
No safety problems have been reported in any the trials conducted to date and some of the more advanced studies have reported improvements in vision. But questions remain. Will gene therapy deliver a permanent cure or something less? What diseases can it treat? All of the currently ongoing trials are for posterior segment diseases – can anything be done for anterior segment disorders? And what will it cost per patient? Alipogene tiparvovec treatment may cost as much as $1.6 million per patient (4) but the extent of its worldwide market numbers in the hundreds – this is whole orders of magnitude smaller than potential market size of the ophthalmic diseases currently under investigation. What’s interesting is that again, in general, Big Pharma aren’t funding the trials; it’s academic centers and biotech firms that are – and it’s likely that pharmaceutical companies will acquire or license the successful agents and market them; an expenditure they expect to recoup. Whatever the eventual costs are, it looks like we may see the first gene therapies for ophthalmic diseases within the next 5–10 years. If these interventions are successful, that’s something to celebrate.
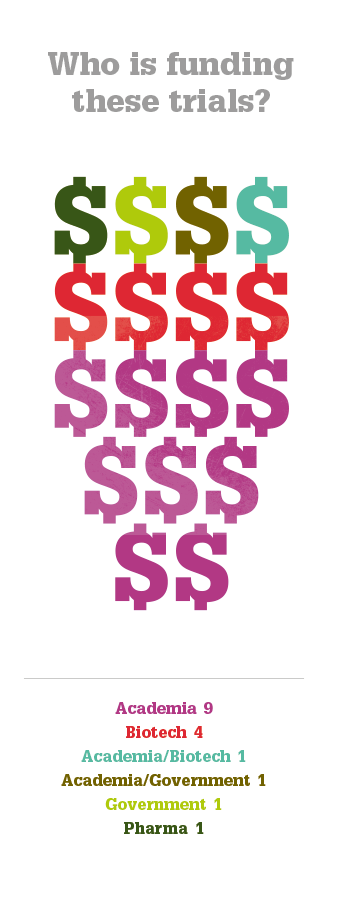
References
- European Medicines Agency, “EPAR summary for the public. Glybera (alipogene tiparvovec)”, October 25th, 2012.
- Human Stem Cells Institute Press Release, “HSCI receives approval to market Neovasculgen – the first Russian gene-therapy drug for treatment of peripheral arterial disease”, December 7th, 2011.
- S Pearson, H Jia, K Kandachi, “China approves first gene therapy”, Nat Biotechnol., 22(1), 3-4 (2004).
- J Whalen, “Gene-Therapy Approval Marks Major Milestone”, Wall Street Journal, November 2nd, 2012.
