At my practice, I see approximately 50 to 60 private and public patients – most with glaucoma – every day. Ergonomics plays a huge role for both me and my patients. And maintaining quality while efficiently managing time is also crucial. In short, I need a streamlined practice that allows me to see all my patients in a comfortable setting, with enough time to discuss the important aspects of their disease, treatment options, and other elements.
Measuring IOP is a fundamental part of my practice – and of any ophthalmic examination – so when planning the workflow, I consider the points above about comfort and time carefully. The patient is usually seated when their IOP is being measured; but occasionally, for example, with bedridden patients or examinations of children under general anesthesia, measurements must be taken with the patient lying down, which presents additional challenges.
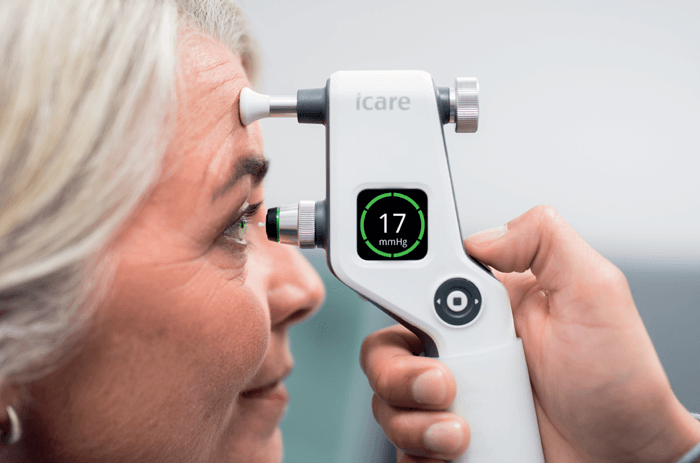
For my practice, I chose versatile iCare rebound technology tonometers – currently the iCare IC200. We were certainly early adopters of this technology, but only because I was convinced of its utility the moment it became available. Over the years, the iCare tonometers have been modified to improve their use, with additional features like indicators of correct alignment, where a green light shows when the tonometer is correctly aligned with the corneal apex (a red light comes on when it is not). Also, the corneal distance indicator shows whether the tip of the probe is too close or too far from the cornea. All of these optimizations give me a high level of trust in the reliability and accuracy of any measurements taken.
We currently work interchangeably with iCare’s IC100 and IC200 tonometers, and we also have patients using the iCare HOME2 model that allows them to monitor their IOP remotely. All these models perform well in our workflow and they are very reliable under all circumstances.
Reliant rebound
Automatic calibration of the tonometer and reliability in measurement indicators make the process very simple – and that means measurements can be made by a range of healthcare professionals (ophthalmologists, residents, optometrists, and others) with different levels of experience, improving the workflow and taking pressure off an already busy ophthalmologist. The fact that no anesthetic or fluorescein is required gives the tonometer a broad spectrum of use.
There are many situations where my team only uses rebound tonometry. In my opinion, these devices are good substitutes for the Goldmann tonometer, yielding similar results, and they have a clear advantage over air-puff tonometry: namely, portability. I have noticed small differences when comparing measurements with those from applanation tonometers, but I believe they may be the result of poor Goldmann tonometer calibration rather than the inaccuracy of the rebound tonometer. In any case, these differences are usually consistent, so it is easy to predict the Goldmann pressure based on the rebound tonometer measurement.
Over the years, I have found iCare tonometers to be reliable, very fast, and especially useful for some patients, such as postoperative cases where contaminated fluorescein drops can cause infections. They have also changed our management of pediatric patients, with or without glaucoma, as it is much easier to obtain a measurement than with applanation tonometry. We can now measure IOP in children as young as a few months, without the need for examination under anesthesia – that’s in stark contrast to applanation tonometers that demand the instillation of a drop or the opening of eyelids.
Cutting Out COVID-19 Cross-Contamination
iCare rebound tonometers enable measuring IOP in a way that is free from air puffs, microaerosol drop formation, and anesthetic drops, which greatly reduces the risk of cross-contamination – no mean feat during a pandemic. iCare’s tonometers are also one of the only handheld tonometers that use safe, disposable probes, protecting patients from transmitting infectious diseases. These single-use, hygienic probes are individually packed and allow for dropless IOP evaluation, protecting patients from contamination. They are also very easy to use for eye care professionals.
Improving eye care
The risk of cross-contamination has always been a problem with applanation tonometers, notably, the disposable probes of iCare tonometers completely eliminate any such risk. The distance between the examiner and the patient is also increased when using rebound tonometers, which further decreases the risk of viral infection transmission. With COVID-19 still being a global health concern, this advantage cannot be taken as lightly as it perhaps was in the past! During the hardest times of the COVID-19 pandemic, rebound tonometry was an important ally in our practices. And in their guidelines for safe practice during the easing of COVID-19 control measures, Spanish ophthalmology societies recommended that air-puff tonometers should not be used to avoid the formation of aerosols that could potentially spread the disease; applanation tonometry was also not suitable due to the contact with the corneal surface and the close proximity to the patient (1).
Final words
I wholeheartedly recommend iCare tonometers to my colleagues; I think they are essential in our practices today. I encourage everyone to try them – for I am sure that they will not look back!
Perhaps more important than my own personal view is that patients are happy with my team using iCare tonometers, which offer quick and painless measurements without the need for anesthetic. Moreover, it is possible to show the patient their pressure, which really helps strengthen the doctor-patient relationship and can improve compliance and adherence.
References
- JA Gegúndez-Fernández, “Spanish Ophthalmology Societies. Recommendations for ophthalmologic practice during the easing of COVID-19 control measures,” Acta Ophthalmol,
