
Allen C. Ho
We’ve experienced a sea change over the last few decades in our ability to treat chronic retinal diseases such as age-related macular degeneration (AMD) and diabetic retinopathy (DR). Although we may not always think of it explicitly, neuroprotection is central to these treatment efforts, as the retina is, of course, a neural tissue. In this context, neuroprotection refers to strategies that aim to preserve the structure and function of retinal cells, not only by supporting the health of nerve cells but also by inhibiting processes like oxidative damage and cellular apoptosis.
Science is now at a point where a variety of both direct and indirect neuroprotective treatment methods are being pursued. Running alongside neuroprotection, there are also efforts toward neuroenhancement, which seeks to improve the performance of existing photoreceptors and other neural cells, and neuroregeneration, which involves cell transplant or replacement.
It’s exciting to have these multiple “shots on goal”, all using different mechanisms to achieve the same end: preserving the visual potential of the neural retina.
Neuroprotection strategies: current methods and future hopes
Inhibition of cellular death pathways
One method that has been explored for neuroprotection of retinal cells is the inhibition of apoptotic, necroptotic, and autophagic cellular death pathways. This approach is being investigated by ONL Therapeutics, who are developing a first-in-class small peptide inhibitor of the Fas receptor (ONL1204, ONL Therapeutics), an upstream activator of cell death and inflammation. This drug, when delivered intravitreally, prevents the activation of the Fas pathway, stopping cell death and inflammation in numerous preclinical models of retinal disease, such as AMD, glaucoma, and retinal detachment (1). ONL Therapeutics is currently initiating a phase II study of ONL1204, to investigate its efficacy in slowing lesion growth and improving visual function in patients with geographic atrophy (GA) secondary to AMD.
Complement inhibition
Modulation of the complement system has also been a focus of recent efforts toward protection of retinal cells in the treatment of geographic atrophy (GA) secondary to AMD, as well as in DR and glaucoma (2). An overactive complement system is implicated in the pathogenesis of GA, resulting in loss of photoreceptors, the retinal pigment epithelium (RPE), and the supporting choriocapillaris. Targeting complement components C3 or C5 has been shown to help control dysregulation of the complement cascade. Intravitreal pegcetacoplan (Syfovre, Apellis Pharmaceuticals), which inhibits C3, and avacincaptad pegol (Izervay, Astellas Pharma), which inhibits C5, are both approved by the Food and Drug Administration (FDA) to slow the progression of GA secondary to AMD (3).
Another intravitreal formulation in development to inhibit C1q – ANX007, developed by Annexon Biosciences – is currently in phase III clinical trials for the treatment of GA, and is noteworthy for having demonstrated not just structural evidence of reduced disease progression, but also preservation of vision (4).
Targeting mitochondria
As mitochondrial dysfunction has been implicated in the pathogenesis of AMD, some therapies targeting mitochondrial function have been investigated for the treatment of retinal disorders. One such therapy currently under investigation is elamipretide (Stealth Biotherapeutics), a tetrapeptide drug which stabilizes the structure and function of mitochondrial electron transport chains. Elamipretide is currently being evaluated in a phase 3 clinical trial in patients with dry AMD (5, 6).
Photobiomodulation (PBM) also targets mitochondria as a means of retinal neuroprotection. In PBM, low-powered lasers or light-emitting diodes are employed to selectively administer wavelengths of visible to near-infrared (NIR) light, ranging from 500 to 1000 nanometers. PBM activates photoacceptors located in the mitochondria to induce biological changes responsible for improving cellular function and vitality. These effects include increased mitochondrial energy metabolism, reduced oxidative stress, and photoreceptor neuroprotection (7).
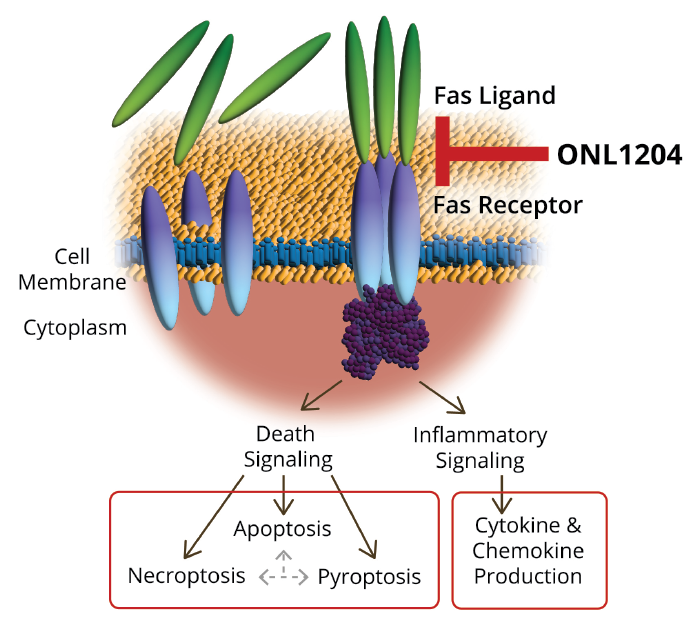
Figure 1. ONL1204 inhibits the Fas receptor-dependent activation of death pathways and reduces production of inflammatory cytokines and chemokines that may be relevant to a variety of diseases such as AMD, glaucoma, retinal degenerations and retinal detachment.
Multiwavelength PBM has been evaluated in dry AMD patients using the Valeda Light Delivery System (LumiThera, Inc) and has shown positive clinical and disease-modifying effects, including improvements in visual acuity and anatomical endpoints (8). Valeda is CE marked in the EU but not yet approved by the FDA. PBM is also being investigated in diabetic macular edema, Stargardt disease, and other retinal conditions (7).
Ciliary neurotrophic factor
Ciliary neurotrophic factor (CNTF) is a strong activator of the JAK/STAT3 signaling pathway, which plays a vital role in mediating inflammation; the use of CNTF has shown particular promise in the treatment of macular telangiectasia (MacTel) type 2, a disease which currently has no approved treatment, and which is generally not responsive to anti-vascular endothelial growth factor (VEGF) therapies (9).
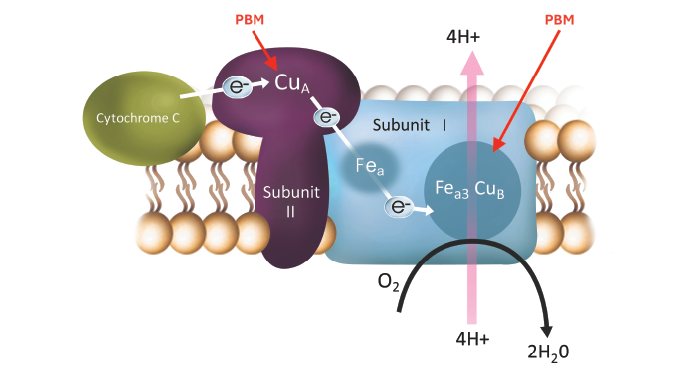
Figure 2. Photobiomodulation utilizes visible to near-infrared (NIR) light to activate photoacceptors in mitochondria to induce biological changes responsible for improving cellular function and vitality. It is CE Marked in Europe and is being assessed by the FDA.
Once considered a vascular condition, we now know that MacTel type 2 is a neurodegenerative disease, where Müller cells, which surround neurons and provide them with nutrition and protection, become dysfunctional, leading to atrophy and disorganization of the outer retina (10). CNTF has been found to induce gliosis in Müller cells, preventing them from undergoing apoptosis, and stimulating the production of photoreceptor survival factors (11).
While several methods for delivering CTNF have been explored, such as a study concluding that mice treated with a recombinant adeno-associated virus vector to express human CNTF gained lifelong protection against photoreceptor degeneration (12), trials testing this technology on humans have yet to be conducted.
Encapsulated cell therapy (ECT) is an innovative approach for delivering CNTF to the retina and the Müller cells (See Figure 1). In ECT, an intravitreal implant houses genetically-modified RPE cells, which produce and deliver therapeutic proteins to the posterior segment—in this case, CNTF (NT-501, Neurotech Pharmaceuticals) (13).
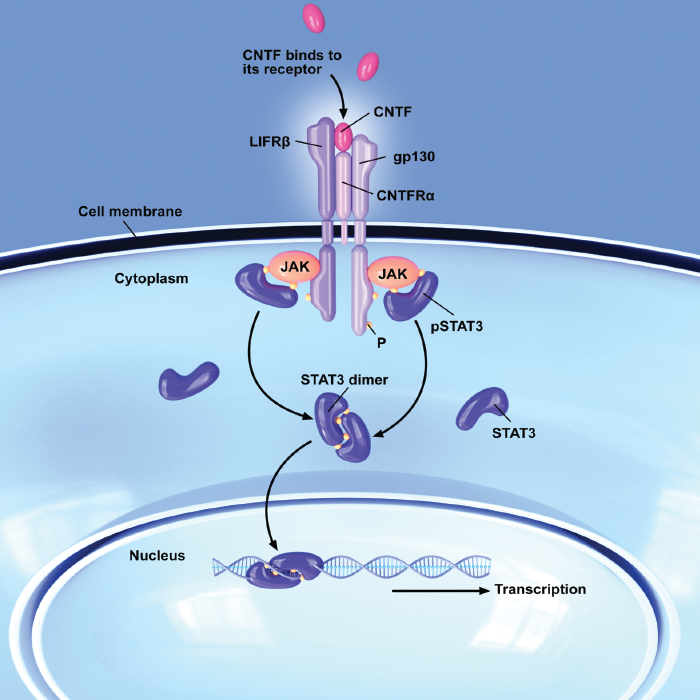
Figure 3. Ciliary neurotrophic factor (CNTF) activates the JAK/STAT3 signaling pathway, which plays a vital role in meditating inflammation and attenuating progression of MacTel.
Encapsulated cell therapy has been investigated in multiple clinical trials. Results from a phase II multicenter randomized trial found that treatment with NT-501 slowed the progression of retinal degeneration in patients with MacTel type 2, and recently presented data from long-term safety studies demonstrated that it is well tolerated (13, 14). NT-501 is currently under Priority Review by the FDA, and if the review is successful, it would represent the first approved treatment for MacTel type 2 (15).
Thinking ahead: evaluating future neuroprotective approaches
Demonstrating a neuroprotective effect can be challenging, especially in chronic retinal diseases that may take years or even decades to progress. As we evaluate emerging therapies that are described as neuroprotective, it will be important to consider how the approach is grounded in science, as well as the safety findings and efficacy endpoints that were studied clinically.
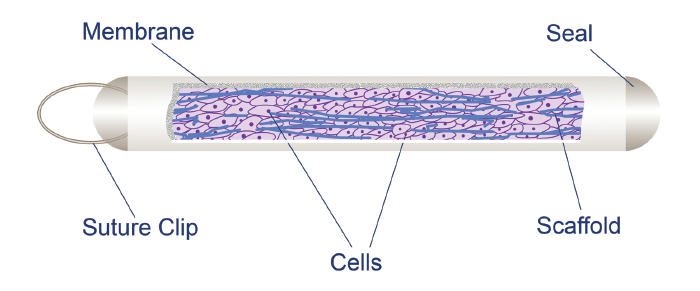
Figure 4. Encapsulated cell therapy (ECT) is an innovative approach for delivering ciliary neurotrophic factor (CNTF) in an intravitreal implant which houses genetically-modified RPE cells. Through a porous membrane, CNTF diffuses into the vitreous, and nutrients pass into the implant to maintain the survival of the cells. Image courtesy of Neurotech Pharmaceuticals.
Ongoing development of retinal neuroprotective therapies will take time and resources, but because vision is so crucial to quality of life, there is great impetus to develop, test, and even combine these therapies. As ophthalmologists, we need to continue to advocate for our patients and work in conjunction with advocacy groups in the space, like the American Academy of Ophthalmology, the Foundation Fighting Blindness, and the MacTel Group, among others.
The anti-VEGF era has been transformational to the field of retina, and it is my belief that we are at the threshold of a new era, where additional major pathways relevant to retinal disease and vision are being studied and positive results are being reported. It will be interesting to see how, in the future, combination therapy approaches – as have been employed in the anti-VEGF space for wet AMD and DR (16, 17) – will be investigated for a greater number of retinal diseases.
I firmly believe that neuroprotective strategies will form an increasingly important aspect of retinal therapies in the near future, and gaining a solid understanding of these practices will be vital to our growth and development as the field continues to evolve.
References
- DN Zacks et al., “Cell death in AMD: the rationale for targeting Fas,” J Clin Med, 11, 592 (2022). PMID: 35160044.
- K Ou et al., “Recent developments of neuroprotective agents for degenerative retinal disorders,” Neural Regen Res, 17, 1919 (2022). PMID: 35142668.
- S Rathi et al., “Therapeutic targeting of the complement system in ocular disease,” Drug Discov Today, 28, 103757 (2023). PMID: 37657753.
- Annexon Biosciences. Annexon to present phase 2 ARCHER Data on protection of vision and photoreceptors with ANX007 in GA at the Retina Society 57th Annual Meeting and 24th Euretina Congress https://ir.annexonbio.com/news-releases/news-release-details/annexon-present-phase-2-archer-data-protection-vision-and
- MJ Allingham et al., “Phase 1 clinical trial of elamipretide in intermediate age-related macular degeneration and high-risk drusen: ReCLAIM high-risk drusen study,” Ophthalmol Sci, 2, 100095 (2021). PMID: 36246187.
- Stealth BioTherapeutics announces first patient enrolled in global phase 3 clinical program for elamipretide in patients with dry age-related macular degeneration. Stealth BioTherapeutics, Inc. Accessed October 1, 2024. Published June 5, 2024. https://stealthbt.com/stealth-biotherapeutics-announces-first-patient-enrolled-in-global-phase-3-clinical-program-for-elamipretide-in-patients-with-dry-age-related-macular-degeneration/
- RC Siqueira, “Photobiomodulation using light-emitting diode (LED) for treatment of retinal diseases,” Clin Ophthalmol, 18, 215 (2024). PMID: 38283180.
- D Boyer et al., “LIGHTSITE III: 13-month efficacy and safety evaluation of multiwavelength photobiomodulation in nonexudative (dry) age-related macular degeneration using the Lumithera Valeda light delivery system,” Retina, 44, 487 (2024). PMID: 37972955.
- F Bucher et al., “CNTF prevents development of outer retinal neovascularization through upregulation of CxCl10,” Invest Ophthalmol Vis Sci, 61, 20 (2020). PMID: 32780864.
- KC Kedarisetti et al., “Macular telangiectasia type 2: a comprehensive review,” Clin Ophthalmol, 16, 3297 (2022). PMID: 36237488.
- W Xue et al., “Ciliary neurotrophic factor induces genes associated with inflammation and gliosis in the retina: a gene profiling study of flow-sorted, Müller cells,” PLoS One, 6, 20326 (2011). PMID: 21637858.
- DM Lipinski et al., “CNTF gene therapy confers lifelong neuroprotection in a mouse model of human retinitis pigmentosa,” Mol Ther, 23, 1308 (2015). PMID: 25896245.
- EY Chew et al., “Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: a randomized clinical trial,” Ophthalmology, 126, 540 (2019). PMID: 30292541.
- C Egan, P Bernstein, “Pooled clinical trial safety data of neurotrophic factor–producing revakinagene taroretcel in people with macular telangiectasia type 2,” Paper presented at 24th European Society of Retina Specialists (EURETINA) Congress, Barcelona, Spain, September 19-22, 2024.
- E Maharjan, “FDA grants Priority Review to novel treatment for macular telangiectasia,” Optometry Times, June 20, 2024. Accessed September 27, 2024. https://www.optometrytimes.com/view/fda-grants-priority-review-to-novel-treatment-for-macular-telangiectasia-type-2
- D Song et al., “Application and mechanism of anti-VEGF drugs in age-related macular degeneration,” Front Bioeng Biotechnol, 10, 943915 (2022). PMID: 36213057.
- TA Bahr, SJ Bakri, “Update on the management of diabetic retinopathy: anti-VEGF agents for the prevention of complications and progression of nonproliferative and proliferative retinopathy,” Life (Basel), 13, 1098 (2023). PMID: 37240743.
