Glaucoma, the leading cause of blindness worldwide, unquestionably requires better treatment alternatives. Ivantis is a company focused on addressing this need. Recently, the Hydrus Microstent, a unique ab interno device, progressed from pivotal trial to FDA approval. Now, in addition to the US introduction, the company is making it commercially available in select international markets.
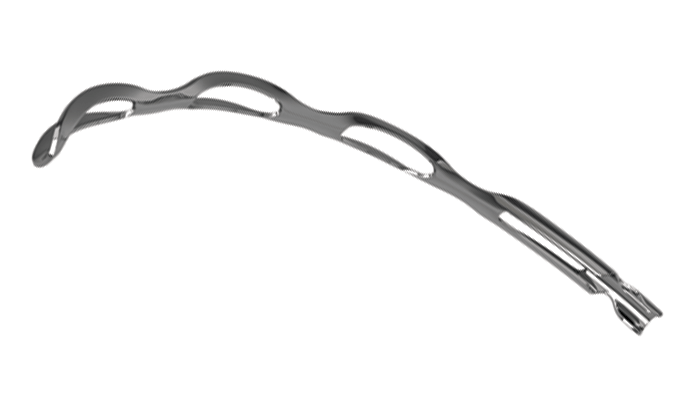
Roughly the size of an eyelash (8 mm in length), the Hydrus Microstent is a novel minimally invasive glaucoma surgery (MIGS) device that has been designed to reduce eye pressure by re-establishing aqueous flow through Schlemm’s canal – the eye’s natural outflow pathway. The Hydrus Microstent is constructed from nitinol, a highly biocompatible material commonly used in a wide range of medical device applications. Its flexibility allows the microstent to be inserted into the trabecular meshwork and placed in Schlemm’s canal, where it acts to restore the eye’s natural fluid flow in three key ways:
- Bypass: The Hydrus Microstent creates a direct connection between the anterior chamber and Schlemm’s canal, thereby allowing aqueous flow to bypass the trabecular meshwork.
- Scaffold: Through dilation and support of Schlemm’s canal, the Hydrus Microstent expands the canal’s cross-sectional area without obstructing fluid access to collector channel ostia.
- Span: The unique reach of the Hydrus Microstent gives 90° coverage of Schlemm’s canal, thereby extending over multiple collector channels; this eliminates the need for precise stent placement, and the need for implantation of multiple devices.
The landmark HORIZON Pivotal Trial was the largest MIGS controlled clinical study ever conducted – it assessed outcomes in 556 mild-to-moderate primary open angle glaucoma patients undergoing cataract surgery. Patients were randomized to receive either cataract surgery plus the Hydrus Microstent, or cataract surgery only (control). Key findings included:
- Over 77 percent of treated patients exhibited IOP reduction of 20 percent or more (less than 58 percent of the control group achieved this level of reduction) – to date, the largest treatment effect reported for any MIGS pivotal trial at 24 months.
- The Hydrus Microstent group achieved more substantial IOP reduction than the control group (7.5 mmHg versus 5.3 mmHg – a 43 percent difference) – the largest difference in IOP reduction reported in a MIGS pivotal trial at 24 months.
- Follow-up showed that 78 percent of Hydrus Microstent patients remained medication-free at two years (as compared with 48 percent of controls) – again, the highest treatment versus control for medication elimination of any MIGS pivotal trial.
This data, together with its expanding history of use in patients – more than 4,000 cases, incorporating a wide range of disease severities – give both surgeons and patients confidence in this new MIGS procedure. At present, Ivantis is focused on marketing the Hydrus Microstent commercially in select markets; increasing levels of favorable real-world data associated with this device – and its unique risk/ benefit profile for treating mild to moderate glaucoma patients – will inevitably see demand grow outside those markets.
