
Launched in 2018, IMCUSTOMEYE is a European consortium developing novel technologies for biomechanical corneal mapping. Responsible for closing the gap between research and commercialization, IMCUSTOMEYE sought to bridge the four critical pillars that serve as the design thinking process behind medical device development: patient, clinic, technology, and the market. This article will shed some light on the challenges and processes IMCUSTOMEYE underwent during the design thinking process and its effort to bridge all four pillars.
1. Context: biomechanics as an unmet medical need
Progressive eye diseases such as keratoconus, glaucoma, and presbyopia develop slowly over time and accumulate in biomechanical changes in the eye. In the early stages, vision loss can be minimal and the biomechanical distortion of the eyes can go unnoticed for a long time before symptom onset. If clinicians are able to assess the specific corneal biomechanics of their patients with objective data, they could improve screening, diagnosis, and treatment outcomes.
Design thinking processes are well established in various industries to realize solutions for particular problems. During the context phase of the design thinking process, it is important to listen. We interviewed leading clinicians from various countries and found that, although they believed that biomechanics are important, a better understanding is needed to establish clinical value. More specifically, clinicians do not need more objective and reliable biomechanical data; what they need is more clarity in interpreting the data. Additionally, clinicians expressed the need for mapping of corneal biomechanical data as ectasia is considered to be a localized weakening of the cornea.
The current standard of care for assessment includes topography, Scheimpflug-based tomography, and anterior segment OCT. Although providing great diagnostic images, these tools cannot assess the individual underlying biomechanical parameters of the distortions in the cornea. Despite diagnostic systems, such as the Corvis ST from Oculus, being able to assess for combined biomechanical parameters, these systems typically only assess limited parts of the cornea (one horizontal meridian). Since biomechanical distortions can occur at any location on the cornea, in order to achieve a full understanding of the cornea, there is still a need for corneal biomechanical data in multiple locations on the cornea.

2. Leadership and Research programme
Identifying the current gap in understanding of corneal biomechanics, The European Commission decided to fund the IMCUSTOMEYE project under the Photonics topic of the Horizon 2020 programme. Over the past four years, IMCUSTOMEYE consortium has been working on novel screening, diagnostic, and treatment planning technologies based on corneal biomechanics. The consortium consists of twelve partners, including research institutes, universities, industry partners, and commercial partners. The universities and research institutes focused on the development of technologies, the industry partners provided the consortium with input from the market, and the commercialization partner (IROC Science) helped to bridge technology, market, clinic, and patient. The project’s leadership, with extensive experience in ophthalmic research and product development, was led by Susana Marcos – an acclaimed researcher in the field of visual optics and ocular imaging. Marcos is known as a pioneer in the development of new techniques for the evaluation of the eye, including retinal imaging instruments, aberrometers, adaptive optics, anterior segment imaging of the eye, and intraocular lens designs.
3. Purpose of the project
The purpose of the project is to improve diagnostic and treatment outcomes by providing biomechanical mapping plus biomarkers which serve as key criteria for diagnosis. Providing clinicians with better biomechanical understanding of their patients’ eyes will improve early disease detection, accuracy, and safety. The project was set up to develop a cost-effective solution for biomechanics-based screening and diagnosis, as well as to investigate ways of using biomechanical data as input for customized treatment planning.
4. The ideation process
Over the course of the project, the team has worked simultaneously on two different attempts to develop a cost-effective technology for biomechanical mapping. Since IMCUSTOMEYE is pioneering understanding of corneal biomechanics, this development process required multiple solution concepts that had to be tested. Here, consortium partners followed in parallel two general concepts: 1) a portable more widely applicable screening tool for early screening, and 2) a larger, full diagnostic system for advanced biomechanical mapping. On top of the screening tool and the diagnostic system, the team developed overarching diagnostic software and treatment planning modules for biomechanical analysis.
The screening device is a low-cost portable device for use both in and out of the clinic, based on sound/ultrasound excitation. The portable screening device ensures that many more pre-symptomatic early stage cases can be detected. Although the device requires more development and tests, the sound/ultrasound excitation method has the advantage of being less invasive for the patient.
The diagnostic device is a full OCT system for in-clinic use, using multi meridian measurements based on air-puff excitation. In contrast to current OCTs in the market that only analyze one meridian, this device analyzes two meridians, providing a highly accurate image of the patient’s cornea with accurate clinical information on more areas of the cornea.
5. Prototype & Test: add-on tool for OCT providing biomechanical mapping
After the ideation phase, our focus was on building prototypes for clinical testing. The goal of this phase was to nail down all we learned to one solution, and then focus on developing this single solution into a commercialisable product. Based on the ideation process, market research, and additional market feedback, the conclusion was that for corneal biomechanics, the best way forward was to deliver an add-on solution that device manufacturers can add to their existing OCT systems. To achieve this, different key components needed to be developed: a) the acoustic stimulus b) the optical detection and c) software to derive and represent the biomechanical factors. It was key to test the different technical solutions during clinical proof-of-concept studies. Within the project, we decided to focus on biomarker sensitivity and specificity for keratoconus patients at different stages.
As a result of positive clinical findings, IMCUSTOMEYE’s add-on modules can be applied to most OCT systems in the market. Providing additional biomechanical mapping of the cornea, the add on module helps to deliver better diagnostics and treatment planning to clinicians – a true plug and play application.
6. Benefits: who is benefiting and why?
With its biomechanical mapping, IMCUSTOMEYE fills the gap in understanding of progressive eye diseases and provides additional biomechanical information for customized treatment planning.
From a clinical point of view, clinicians will have a solution for better understanding the biomechanical state of the cornea. IMCUSTOMEYE’ssolution helps to determine the patient’s safety profile for specific treatments and allows for more advanced treatment planning. This, in turn, benefits patients as it allows diagnosis and customized treatment based on the specific biomechanical eye conditions of each patient’s eye with potentially higher predictability and safety.
From a commercial point of view, OCT companies benefit by having comprehensive biomechanical measurements as an add-on functionality to their existing devices. There is a great benefit of adding biomechanical functionalities to existing devices compared to launching completely novel devices because it avoids most costs related to launching a new product (e.g. production line, marketing, sales costs, etc.). Having a product that is complementary to a manufacturer’s portfolio is a much more attractive proposition than a product that serves as a substitute. Moreover, in Europe, biomechanical measurements are not reimbursed by healthcare programs, leaving patients with high costs for any biomechanics-based diagnosis or treatment. From a practical point of view, the add-on strategy is also easier for clinicians, because they can keep using the same devices as opposed to learning and investing in a completely novel technology.
7. Market
Biomechanical measurements might be of interest for other applications, such as evaluating donor corneas, other medical industries, food tissue assessment, and industrial materials assessment. Despite this, every use case requires tailored product design, specific market knowledge, and access outside the scope of the development programme...
As a result, one has to evaluate the market access potentials within this focus area. In broad terms, corneal biomechanics are of clinical relevance in detecting and diagnosing corneal ectasia at an early stage and in providing intraocular pressure measurements with high accuracy. Besides this, using biomechanical data in planning therapeutic interventions, such as corneal refractive laser surgery, corneal incisions for cataract surgery, corneal cross linking, or even contact lens fitting have been considered to be of clinical relevance for decades. Although the laser refractive and cataract market are the most attractive areas, it is difficult to provide clinical evidence of superior outcomes in such surgical interventions as it requires large numbers of sample sizes to demonstrate statistical relevance. In contrast, diagnosing corneal ectasia and evaluating the outcomes of corneal cross linking are more directly related to the biomarkers and, therefore, provide better clinical relevance.
The developed solutions successfully completed an initial clinical proof of concept to diagnose early stage keratoconus and demonstrated the ability to measure the effect of corneal cross linking, tested in clinical evaluations at different clinical sites in the stage of clinical validation.
As a result of this market evaluation, the first application has been developed and tested for keratoconus, making the keratoconus segment the first target segment. Next market segments will be laser refractive surgery, laser cataract surgery, glaucoma diagnosis and surgery, and contact lenses. The next step is to start industrial design and regulatory approvals and find the right commercial partners. The key for a market entry is also the intellectual property that protects the technology and the product; one has to understand the freedom to operate in the market. This is often underestimated when new technology is developed that potentially bears a high risk for market entry. Therefore, we conducted an FTO early in the project to guide the research and development efforts into areas that overcome such hurdles.

8. Lessons learned and key take home messages
During the course of the project, the consortium went through a design thinking process that included evaluating different technologies to solve a particular problem, refocusing on the most promising solution, and exploring a wide range of markets and market entry options. This process came with both significant achievements and lessons wrought from unexpected challenges. Working with a consortium of 12 members spread over five countries brings together some great and diverse minds. During regular meetings, perspectives were voiced on the table that challenged all team members to think outside of the box. A big lesson was taught about the challenges in alignment of all consortium members. Ensuring that everyone was working with a common objective in mind was a critical element to establish. The design thinking process helped by guiding everyone from the wide area of possible applications towards the most promising solutions. Alignment here does not just refer to alignment of organizational matters, but also to the alignment of perspectives, especially when clinicians, researchers, and commercial partners work together.
Scientific researchers are generally highly focused on discovering novel scientific innovations, but they do not approach this process from a product development perspective. Although this scientific approach is often the foundation for the best discoveries, design thinking is focused on bringing a product to the market that fulfills market needs. Therefore, market needs must be the ultimate driver of the development of solutions. Although clinicians rightfully focus on the clinical benefits of a new technology, they are usually less aware of the challenges in research, product design, and regulations. In addition, when performing market research and interviews with clinicians one has to consider not only such clinical aspects. Other factors such as ease of use or return-on-investment can make the difference between buying or not buying a future product in the market. Industry members are focused on how to create value and commercial success. It is therefore important to engage future potential partners, licensors, or acquirers of a technology into the development process to not only appreciate market trends, but also the specific requirements of such companies to implement the technology into their product portfolio.
It needs to be understood among all team members that a future owner of the solutions requires not only the patents to commercialize a product. In most cases the technology documentation and the key know-how elements are of ultimate importance when a technology is licensed or acquired. A challenge that needs to be addressed early in a multidisciplinary, multi-site research and development project is the seamless transfer of such knowledge towards a new owner. Therefore, it needs to be clear who is responsible for representing, negotiation and transferring the knowledge within the consortium. Potential conflicts of interest among the consortium members must be dissolved early in the process so that the team represents a coherent and aligned group towards a potential acquirer of the technology. One solution can be a spin-off company or a joint-venture structure that represents the interests of the inventors and the consortium.
In conclusion, it is clear that by bringing all different viewpoints into one direction, a team can achieve a successful innovation project.
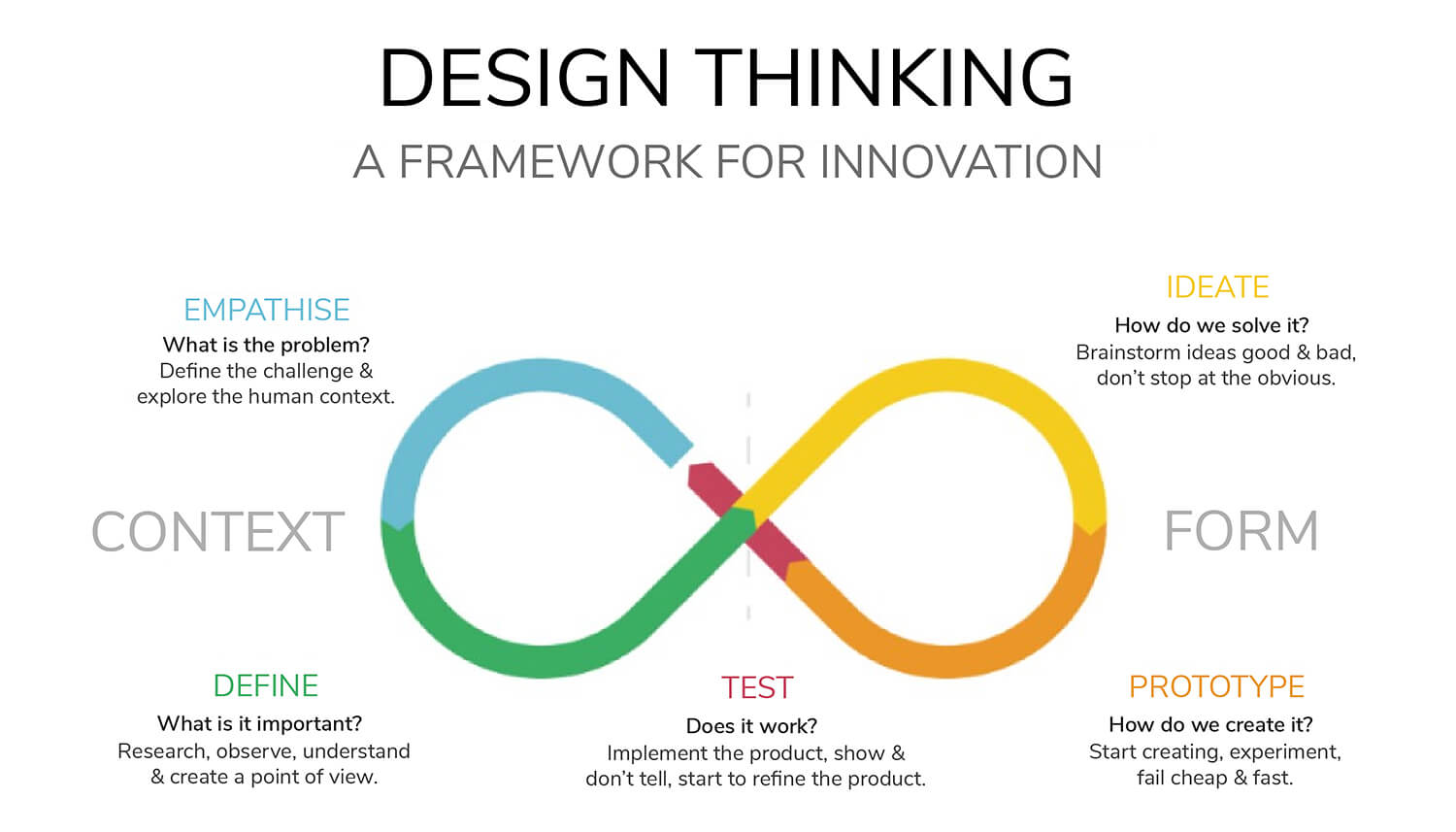 A visualization of the design thinking process (Image courtesy of Michael Mrochen & Lisa Kleintjens)
A visualization of the design thinking process (Image courtesy of Michael Mrochen & Lisa Kleintjens)
