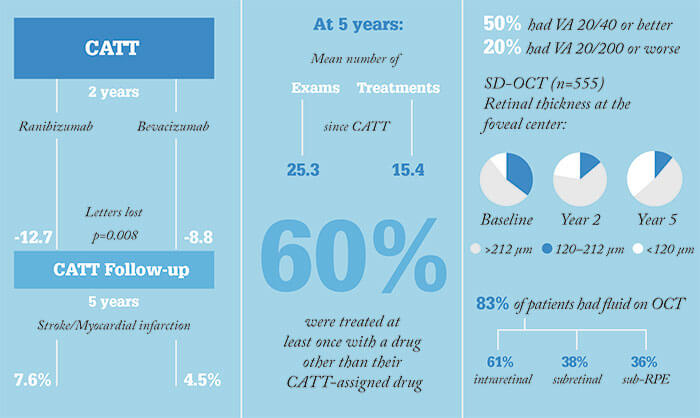
At the time of its announcement, the Comparison of AMD Treatments Trials (CATT) trial caused quite a stir: it was a rare case of a large clinical trial funded not by industry, but by the US National Institute of Health. Large-scale clinical trials of anti-VEGF agents for retinal disease also tend to focus on the first one to two years – relatively few investigators have characterized patients’ outcomes after four or more years. The CATT investigators have. Even though the trial ended after two years of follow-up (1), all patients that were alive at the end of the study were targeted for inclusion in the CATT Follow-up study (2). Three years after being released from the CATT trial protocol, patients were called back for examination. This is what the investigators found:
No statistically significant differences in VA or morphologic outcomes between drug or regimen groups were observed after five years, other than one observation that between two and five years, the patients originally assigned to ranibizumab during the two years of the CATT study lost more letters than the bevacizumab group (-4 letters, p=0.008). Regarding safety outcomes, the original ranibizumab patient group had a higher incidence of stroke and heart attack (7.6 percent vs. 4.5 percent; p=0.04), but for both this difference, and the difference in letters lost between years two and five, the results need to be interpreted with caution: patients were able to switch drugs or use other treatments after two years in the trial. The good news was that half of all patients retained a VA of 20/40 or better (meaning that in the US, they’re not disqualified from driving cars on the basis of their vision) – and that one in 10 had 20/20 vision. The bad news is that two in 10 patients had 20/200 vision or worse. The hope is that ongoing research will soon yield therapies that are able to help them too.
References
- DF Martin et al., “Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results”, Ophthalmology, 119, 1388–1398 (2012). MG Maguire et al., “Five-year outcomes with anti-VEGF treatment of neovascular age-related macular degeneration (AMD)”, Ophthalmology (2016). [Article in press].
