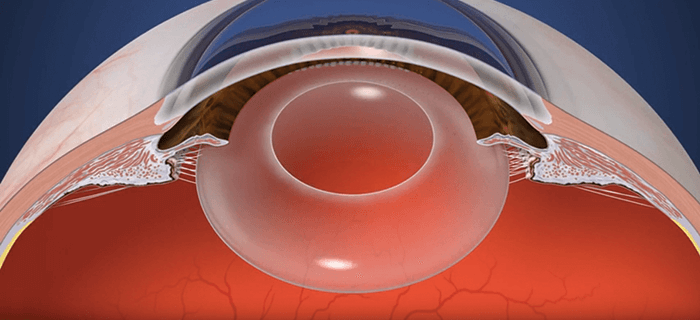
- Most cataract surgeons usually see an elastic capsular bag during a cataract procedure – retinal surgeons see a stiff, fibrosed bag months to years later
- The fibrosed bag, sectioned appropriately, can actually help the right IOL vault forwards and backwards in response to ciliary muscle tension
- Primate studies suggest that this approach results in IOL accommodation-disaccommodation similar to that of the crystalline lens
- Getting an accommodative IOL right has the potential to be transformative, if only we can achieve true accommodation
When it comes to cataract surgery, I have a different perspective on the procedure: I’m a retinal surgeon. When I see an IOL in the capsular bag, it’s months or years after it was implanted and it’s no longer the elastic item cataract surgeons interact with. Instead, I usually see something that’s encased in a rigid and fibrosed disc. The lens is, effectively, straight-jacketed, so any chance of ciliary muscle-induced accommodation is long gone. I always thought that this was a shame – and the observation stuck with me. Might this be something that a simple solution could fix?
I discussed this with a friend who, at the time was an IP attorney, but who had been a nuclear physicist and ophthalmologist in prior careers, who connected me the head of the College of Nanoscale Science and Engineering institute at SUNY Poly in Albany, New York, in order to start a brain trust for innovation. We started to bounce around some ideas; he thought we should develop some advanced technology like a retinal prosthesis, but I suggested something simpler, a mechanical device like an accommodating IOL. Surely if we could get the design right, this might be a solution. The meeting didn’t go anywhere back then, but it didn’t stop me from wondering how this issue of rigidity might be overcome, and indeed what role other factors might play – nanofibers, materials, the capsular bag, elasticity and accommodation, and ultimately, zonular capture haptics.
The birth of a concept
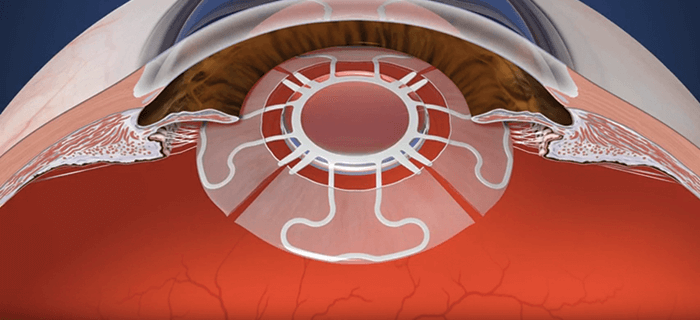
My thinking was this. The fibrosed capsular bag restricts movement. But if it was cut into sections (with radial capsulotomies), then each section is able separate from the others during disaccommodation. When the ciliary muscles contract and relax, this should mean that the IOL (with appropriate, flexible haptics) could vault forwards and backwards, respectively. Embracing the fibrosis further, we could utilize it to attach zonules to the individually mobile haptics like Velcro. And so the zonular capture haptics concept – and idea of how to design an IOL that could utilize it – was born. I drew my concept and had it notarized, and then almost forgot about it. But two years later, I decided to pursue it. As this was not my field at all, I went to an ISOP meeting in Barcelona in 2009 to hear what the presbyopia and cataract specialists were working on. None voiced similar ideas to mine – so this made me confident that my idea was unique. I came back home and filed a preliminary patent application with a friend of the family who was a patent attorney. My daughter helped make a conceptual animation for me in college and my son, who was a professional artist, made illustrations. I then contacted Alcon and presented my concepts to them. The company liked my idea and encouraged me to get back in touch when I had some results.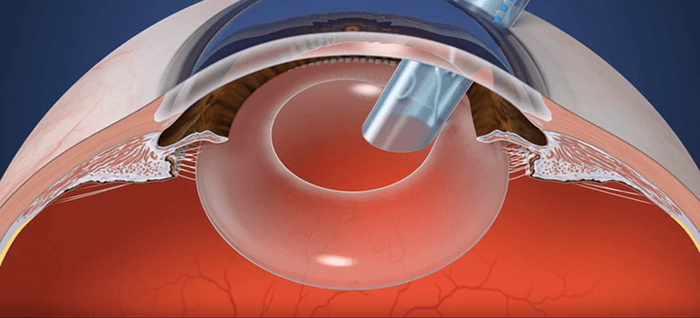
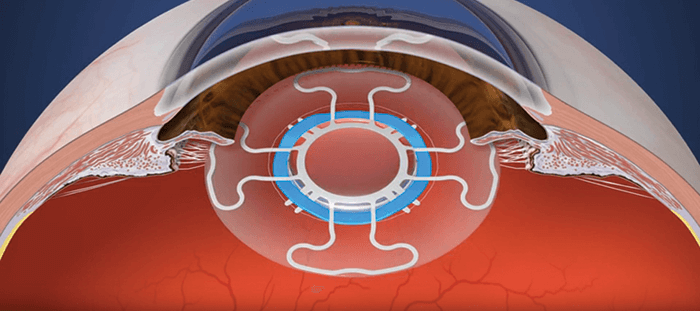
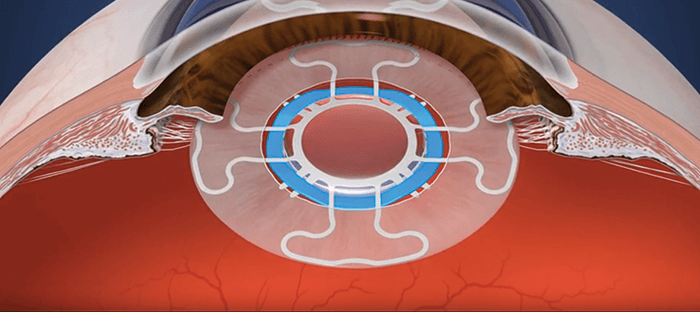
An idea only gets you so far
It was then when I realized that just having a good idea is absolutely not enough. So Z Lens LLC was incorporated, and then things really got moving... I visited Paul Kaufman and Mary Ann Croft in Madison, Wisconsin, as they are leaders of one of the best presbyopia research groups in the US. I presented my idea to them and proposed a proof-of-principle study. They were intrigued, so much so that they were interested in doing an animal study. I paid for the first animal study out of my own pocket. We implanted a handmade ring haptic structure that I made from surgical Prolene in two primate eyes, let the capsular bag fibrose, whereupon they sectioned it and measured changes in the haptic upon accommodation. The handmade haptic dilated and constricted almost 1:1 with the ciliary body, for over a year after implantation (see Box “Proving the Point”).A Proof of Principle, Unoptimized Z Lens IOL Prototype
One of our early animal experiments involved implanting a “proof of principle” prototype of the Z Lens IOL concept into two Rhesus monkey eyes. This was a simple, un-optimized IOL with a “borrowed” 5 mm optic and Prolene haptics. After waiting three weeks we sectioned the fused capsular bag. Two weeks after that, we performed ultrasound biomicroscopy (UBM) and plano perfusion lens and OCT imaging. Each time, we induced pharmacological accommodation via corneal iontophoresis of 40% carbachol in agar – a supramaximal dose for inducing accommodation. What we found was that the pharmacological stimulation of accommodation yielded an average maximum accommodation of 4 D, which exceeded expectations, and both animals reached maximum accommodation by 10 minutes after carbachol administration. We observed a rapid return to near-baseline refractions after only 20 minutes.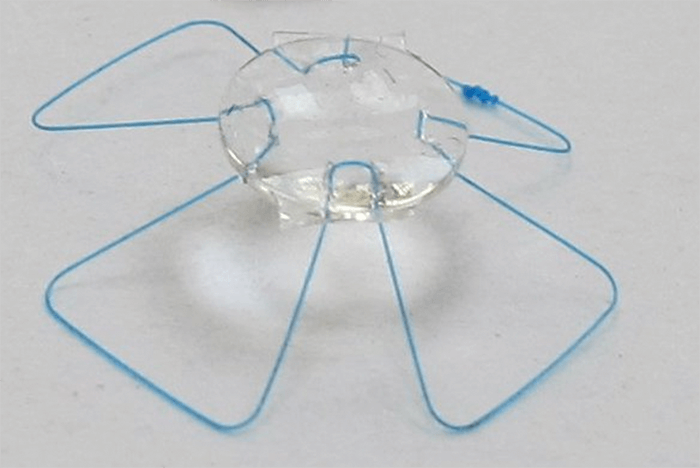
The in vivo Zonular Capture Haptic Dynamometer
Prolene is not an appropriate haptic material for a dynamic IOL. We designed and built a haptic structure from a super elastic, shape memory alloy that could cycle between accommodated to disaccommodated shape over millions and millions of cycles without loss. We have repeated the experiment with this device and we used it as an intraocular, in vivo dynamometer. The data from this dynamometer allowed us to measure the actual forces exerted by the eye in vivo, on a fibrosed, post-surgical capsular bag, and optimize the haptic structure force response curves.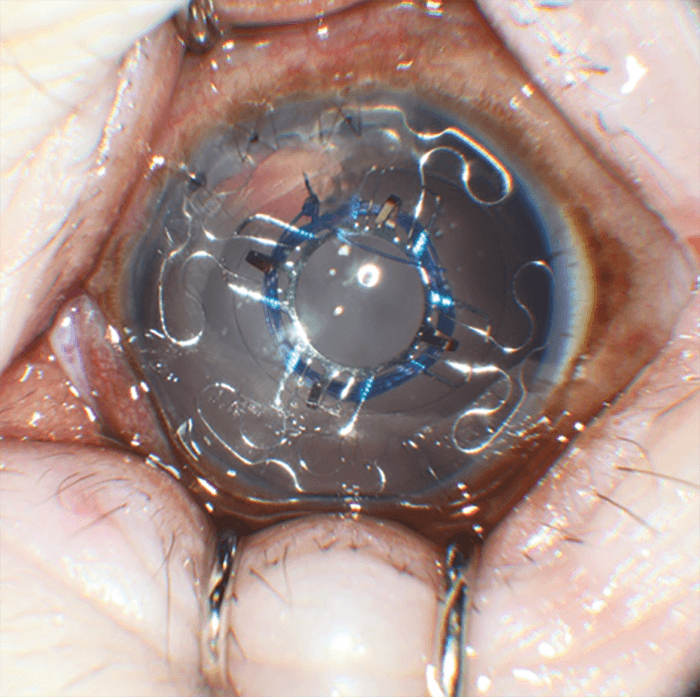
The Optimized Zonular Capture Haptic
We then tested the optimized Zonular Capture Haptics. As you can see, it integrates perfectly with the fibrosed capsular bag and it produced an axial shift of 0.76 mm on OCT, as seen. When we used a physiologic level of accommodation, via stimulation of the mid brain Edinger-Westphal (EW)nucleus, this optimized structure matched the movement dynamics of a young crystalline lens.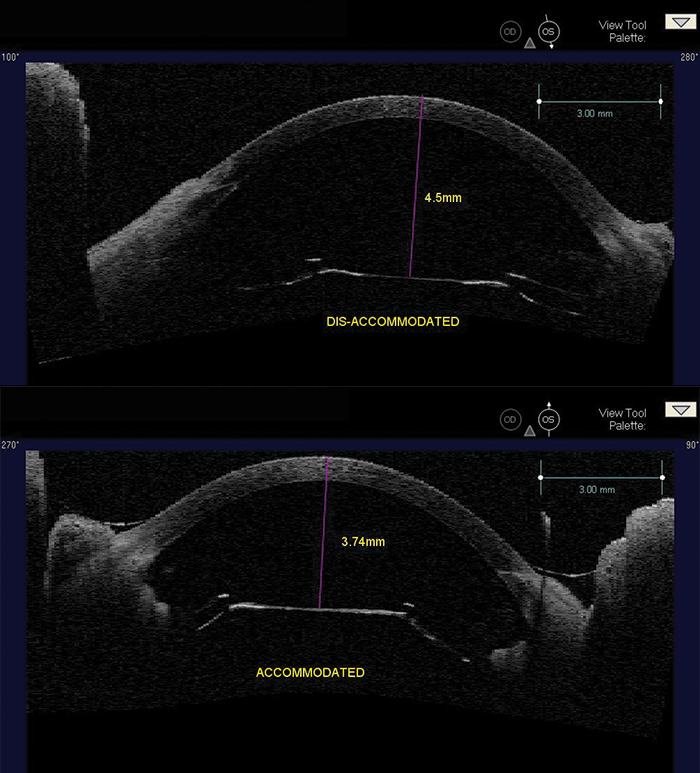
AD-IOL with Zonular Capture Haptics
The next experiment was to show that an integrated accommodating-disaccommodating (AD-) IOL, using the optimized haptic and custom made optic, could provide physiological levels of accommodative movement (in primates). Accommodation was achieved through either electrode stimulation of the EW midbrain nucleus or carbachol administration, and we assessed optic axial shift, haptic flexion, and refractive change by OCT, UBM, Scheimpflug imaging, and Hartinger objective refraction.
Here’s what we found. When we compared the animal’s crystalline lens in the same eye, using EW stimulation, before and after surgery, the crystalline anterior lens face shifted by 0.48 mm, and the anterior optic face by 0.47 mm (To view the Z Lens IOL undergoing EW-stimulation, visit: http://top.txp.to/ZlensIOL). In a different animal eye, seen below, the axial shift of the AD-IOL actually exceeded the axial shift of the crystalline lens.
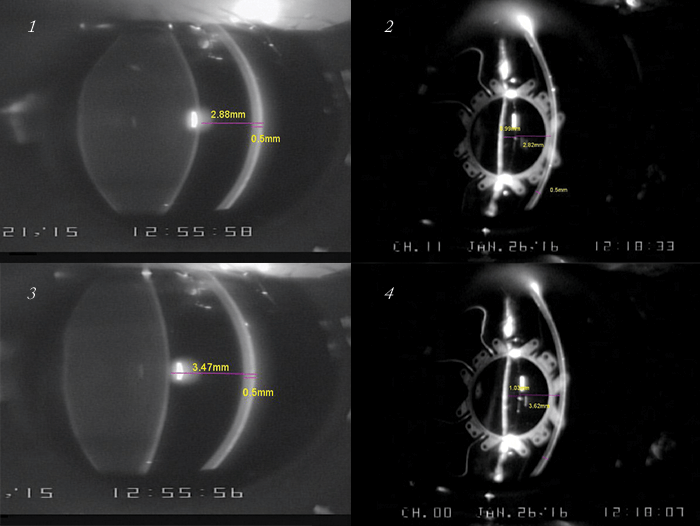
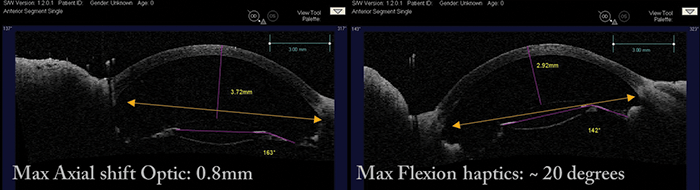
One year after implantation, we found that we could produce a maximal axial shift in the optic of 0.8 mm and a maximal haptic flexion of 20°. Given that the biometrically predicted accommodation of the Z Lens IOL was 1 D per 1 mm of axial shift, we actually observed (using Hartinger objective refraction) a mean accommodation of ~2 D using electrode stimulation, and up to 4 D using carbachol – and this meets the FDA requirements for an accommodating IOL label.
But I didn’t have unlimited funds and primate studies are extremely expensive, so I inquired about government grants. During this time, I was introduced to the serial entrepreneur, Ted Eveleth, who became my partner, and has been the financial arm of Z Lens LLC ever since. Ted was an experienced grant writer – among many other things – and he helped secure all of the grants that allowed us to get as far as we have without any stock dilution. We recruited advisors: Tom McNicholas and Tom Dunlap (who have reputations that precede them), and the designer of our IOLs Rob Stupplebeen, a former Bausch + Lomb engineer. Dave Dudzinski, an incredible engineer at the Learner Research Institute at the Cleveland Clinic made all of our recent prototypes. They’ve all been huge assets to our company.
Fine-tuning the process
We’ve now refined the process (see Box: The Z Lens IOL implantation and activation steps): after capsulorhexis and phacoemulsification, the Z Lens is placed into the empty capsular bag – with a restraining device that holds it in a flat configuration. The surgery is then completed following normal procedures, then we wait. The lens acts like a traditional monofocal lens... but after a few weeks, the capsular bag becomes stiff and prevents movement. To restore movement, we activate the Z Lens by cutting the capsular bag in between the haptics and releasing the restraining device – and this can be done non-invasively with a YAG or femtosecond laser. The radial capsulotomies (also made with the laser) enable the bag to move once again in response to ciliary muscle movement – and with it, the Z Lens. In other words, the Z Lens now mimics the movement of the natural lens by flattening out during disaccommodation and bolting forward during accommodation (Figure 1). We’ve now completed the animal work with our first-generation Z Lens IOL (see Box “Proving the Point”). We have more than a year’s worth of data in nine animal eyes: an accommodating-disaccommodating IOL with a single rigid optic that would meet FDA label requirements, and is ready for resizing for human eyes.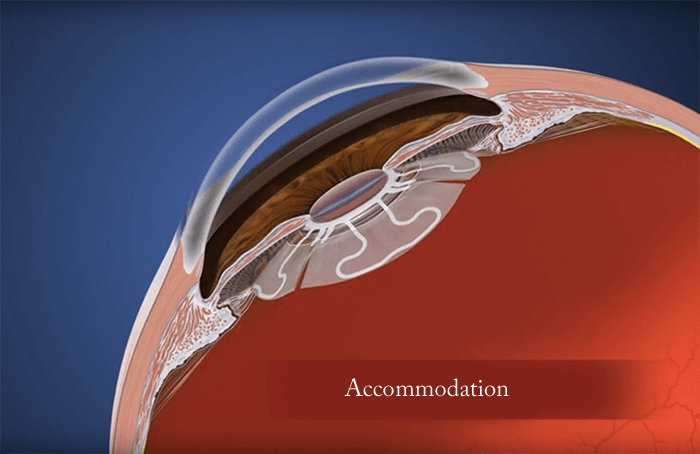
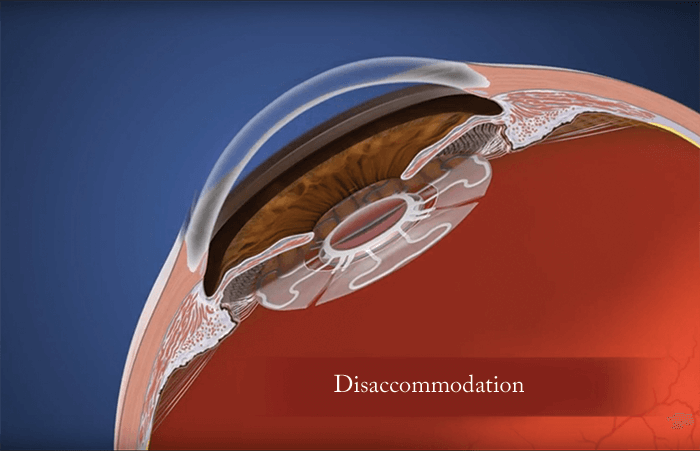
One more thing. The crystalline lens doesn’t just move backwards and forwards during accommodation and disaccommodation: it changes shape too. We now have a dual mode IOL in development that adds a shape-shifting optic to provide more accommodation, and in silico projections suggest it’s capable of an accommodation range of 10 to 14 D, and we expect to be testing prototypes in primates very soon. The accommodative IOL market is potentially worth $0.5 billion, and this will only increase – if we can offer true accommodation. With the work that we’ve completed so far and what we have in the pipeline, it’s something that we hope to show in the not too distant future… In addition to being the inventor of the Z Lens IOL, Paul Beer is a Professor of Ophthalmology at Albany Medical College, has received multiple teaching awards, merit awards from the AAO, ASRS, and was appointed the principal investigator for 25 multicenter clinical trials and conducted multiple investigator-sponsored trials.
Here’s what we found. When we compared the animal’s crystalline lens in the same eye, using EW stimulation, before and after surgery, the crystalline anterior lens face shifted by 0.48 mm, and the anterior optic face by 0.47 mm (To view the Z Lens IOL undergoing EW-stimulation, visit: http://top.txp.to/ZlensIOL). In a different animal eye, seen below, the axial shift of the AD-IOL actually exceeded the axial shift of the crystalline lens.

One year after implantation, we found that we could produce a maximal axial shift in the optic of 0.8 mm and a maximal haptic flexion of 20°. Given that the biometrically predicted accommodation of the Z Lens IOL was 1 D per 1 mm of axial shift, we actually observed (using Hartinger objective refraction) a mean accommodation of ~2 D using electrode stimulation, and up to 4 D using carbachol – and this meets the FDA requirements for an accommodating IOL label.

One year after implantation, we found that we could produce a maximal axial shift in the optic of 0.8 mm and a maximal haptic flexion of 20°. Given that the biometrically predicted accommodation of the Z Lens IOL was 1 D per 1 mm of axial shift, we actually observed (using Hartinger objective refraction) a mean accommodation of ~2 D using electrode stimulation, and up to 4 D using carbachol – and this meets the FDA requirements for an accommodating IOL label.
One year after implantation, we found that we could produce a maximal axial shift in the optic of 0.8 mm and a maximal haptic flexion of 20°. Given that the biometrically predicted accommodation of the Z Lens IOL was 1 D per 1 mm of axial shift, we actually observed (using Hartinger objective refraction) a mean accommodation of ~2 D using electrode stimulation, and up to 4 D using carbachol – and this meets the FDA requirements for an accommodating IOL label.
