The Uniqueness of Uveal Melanoma
Uveal and skin melanoma originate in the same type of cell, but are very different in terms of molecular mechanism, pathobiology, prognosis and therapeutic strategy. Here, the features of uveal melanoma are described and the latest therapeutic developments discussed.
At a Glance
- Uveal and cutaneous melanomas have distinct genetic origins and require dramatically different treatment strategies.
- If uveal melanoma metastasizes, it typically spreads to the liver. Surgical resection and chemotherapy usually are not options in such cases.
- Hepatic chemoembolization is one current treatment option, but a number of new approaches are currently under evaluation.
- New targeted therapies under development include inhibitors of inhibitors of the c-MET receptor (overexpressed in metastatic uveal melanoma tumors) and the kinase, MEK (implicated in uveal melanoma tumor growth).
It starts with a mole. Like cutaneous melanoma, the first sign of melanoma of the eye – or uveal melanoma – is a nevus or spot on the choroidal wall, iris, or ciliary body. It’s estimated that 5 to 10 percent of the general population have nevi in their eyes (although that incidence is much lower in people with dark-colored irises), but thankfully fewer than around one in ten thousand nevi will develop into ocular melanoma (Figure 1).
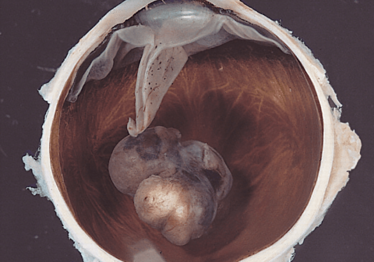
Figure 1. An example of uveal melanoma: a variably pigmented, mushroom-shaped choroidal tumor that has ruptured the Bruch membrane and grown into the subretinal space.
As in cutaneous melanoma, the pigmented lesions in the eye need to be regularly monitored for changes in shape and size. However, uveal melanoma is a much different disease from the cutaneous version, both in terms of genetic characteristics and how it spreads. Cutaneous melanoma is characterized by mutations in the genes BRAF and NRAS, whereas uveal melanomas lack these cardinal mutations. Instead, among other mutations, 80 percent of cases carry alterations in GNAQ or GNA11, members of the G-protein coupled receptor superfamily (Figure 2). In addition, although cutaneous melanoma is often linked to sunlight, there is surprisingly little evidence to connect UV exposure and melanoma of the eye.
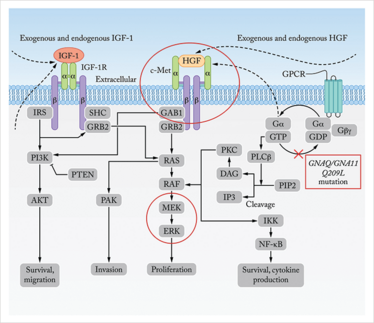
Figure 2. The Paths to Metastasis in Uveal Melanoma.Present in nearly 80 percent of uveal melanomas, mutations in GNAQ and GNA11 (boxed) constitutively activate the RAF/MEK/ERK pathway, which promotes proliferation, while also increasing cell survival and cytokine production via the NF-κB pathway (far right). In addition IGF-1 and HGF, exogenous signals produced by the liver, also activate the RAS, PAK and P13K-mediated pathways to increase cell migration, invasion and proliferation. A novel clinical trial combining two drugs aims to decrease chance of metastatic disease by blocking c-Met (or the HGF receptor) and signaling from the liver, while also inhibiting MEK and ERK to inhibit constitutive activation by the GNAQ and GNA11 mutations (circled). Adapted from reference 11.
When uveal melanomas metastasize, those originating in the eye tend to be far more aggressive and resistant to standard forms of therapy than those originating in the skin. It’s a relatively rare disease – about 2,000 Americans are diagnosed with ocular melanoma each year, according to the Melanoma Research Foundation – therefore it has not had the attention and research funding that many other cancers receive.
Despite these facts, over the past 20 years, the field of uveal melanoma has made tremendous progress in recognizing the disease, treating the primary tumors in the eye, and developing new ways to attack metastatic disease.
In collaboration with Jerry and Carol Shields at the Wills Eye Institute in Philadelphia, Each year, Wills Eye Hospital treats approximately 600 new patients with uveal melanoma and Thomas Jefferson University treats approximately 100 new patients with metastatic uveal melanoma. Although the diagnosis of uveal melanoma can be difficult, we have developed techniques to help identify nevi at high risk of converting into melanoma (1). In an examination, ophthalmologists look at factors such as tumor diameter and thickness (2,3), orange pigment on the tumor surface, and subretinal fluid around the base of the lesion to help determine the diagnosis.
If uveal melanoma is suspected, patients undergo a detailed work-up including ultrasound to assess the size of the lesion, optical coherence tomography for detection of subretinal fluid, autofluorescence to allow for orange pigment evaluation, and angiography to visualize the level of blood flow to the tumor (Figure 3).
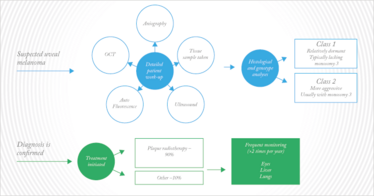
Figure 3. Typical diagnosis and treatment flow for patients with suspected, then diagnosed uveal melanoma.
Once diagnosis is confirmed, the first-line treatment for primary uveal melanoma is usually plaque radiotherapy, a procedure that involves inserting a radioactive device that is surgically attached to the sclera, at a point that precisely overlies the melanoma, in order to deliver a high but localized dose of radiation to the tumor. The procedure is used on about 90 percent of patients with ocular melanoma treated at Wills Eye.
At the same time, a sample of the tissue is taken for genetic and histological analysis. The severity of the cancer can be predicted by testing for monosomy 3 (the loss of a copy of chromosome 3) in addition to abnormality in chromosome 6 and 8. Diagnosis of monosomy 3 indicates a mutation in BAP1 – a chromatin-modulating gene – in one allele of Chromosome 3 and a complete loss of the other copy of the chromosome. Patients with these mutations have a much greater risk of metastatic disease and are rapidly treated with local and/or systemic chemotherapy in addition to plaque radiation. Recently, molecular analysis of tumors (4) has led to the categorization of uveal melanoma into class 1 (relatively dormant and usually lacking the monosomy 3 mutation) and class 2 (more aggressive usually with monosomy 3). After the primary tumor is treated, patients with ocular melanoma remain on frequent systemic monitoring, with checks on their eyes, liver and lungs being performed a couple of times per year (Figure 3).
Although the rates of patients who develop metastases at 10 years vary from 20 percent to 67 percent, depending on the thickness of the primary tumor (2), those that do develop aggressive metastases have a median survival rate of less than one year.
Current Treatments for Metastatic Disease
The gold standard of treatment for many cancers – surgery and chemotherapy – is generally ineffective for patients with uveal melanoma metastases. Multiple metastatic sites are usually found in the liver, making surgery impossible, and uveal melanoma metastases are resistant to traditional chemotherapeutic agents delivered systemically.
For the past 20 years, the first-line approach for patients with hepatic metastases has been chemoembolization. This procedure utilizes high doses of a chemotherapeutic agent, which are delivered directly to the liver via the hepatic artery before the blood vessels that feed the tumor are blocked, or ‘embolized’ in order to localize the dose to the organ with the metastases. The location of the tumor, and the blood vessel that feed it, are visualized by angiography, in which a contrast agent is injected into the blood vessels for X-ray imaging. The chemotherapeutic agent is then injected, limiting systemic exposure and allowing a much higher local dose to be delivered than could be tolerated systemically. The approach has been used with various chemotherapeutic agents, and clinical trial protocols have differed to the extent that it is impossible to say whether one agent shows survival benefit over others: comparative (and comparable) trials using a standard protocol have been lacking.
More recently, we have begun to offer patients immunoembolization using granulocyte-macrophage colony-stimulating factor (GM-CSF), instead of or in addition to chemoembolization. Immunotherapy has shown benefit for cutaneous melanoma and there is hope that targeting the immune system could also improve survival for patients with metastatic uveal melanoma (Table 1). However, the tissue microenvironment of the liver tends to induce tolerance to antigens presented there, most likely because of the organ’s constant exposure to food-based antigens that should be ignored from an immunological perspective. Indeed, the liver contains more than 70 percent of the body’s macrophages as well as other antigen presenting cells, most of which are involved in tolerance induction rather than activation. GM-CSF embolization is intended to break this tolerance by activating antigen presenting cells and providing tumor antigen to the immune system in an inflammatory milieu that activates rather than suppresses local immune cells – in essence, creating an in-situ tumor vaccine.
A Phase I clinical trial with 34 patients with metastatic uveal melanoma that we published in 2008 (5) showed promising results. The overall response rate for tumor shrinkage of hepatic metastases, defined by independent radiologists using the standard Response Evaluation Criteria in Solid Tumors (RECIST) was 32 percent. When we compared similar patients treated with chemoembolization at our institution, those receiving high dose (1500 to 2000 micrograms of GM-CSF) immunoembolization had a longer overall survival (median of 20.4 months for immunoembolization versus 9.8 months for chemoembolization).
We followed this research up with a Phase II clinical trial presented in 2011 (6) comparing immunoembolization with embolization alone. We demonstrated that patients who received immunoembolization had longer time to progression in extra-hepatic sites, compared with embolization alone. We further demonstrated that the degree of inflammatory response, as measured by cytokine release after immunoembolization treatment, is correlated to the time to progression in extra-hepatic organs, which indicates systemic effect of immunoembolization. We continue to analyze the results of this trial.
Newer immunotherapy approaches are currently in clinical trials. For example, researchers are testing agents, such as PD-1 and CTLA-4 blockers, that help counteract tumor immunosuppression, and are also showing success in treating some cancers. In addition, a clinical trial reported last year showed the first benefit of systemic therapy for uveal melanoma metastases using therapy that targeted the MEK signaling pathway (Figure 2). The study reported that 50 percent of patients had some tumor shrinkage, although that included patients with shrinkage of as little as one percent. Using RECIST criteria, 15 percent of patients responded (7).
Replicating the Plaque Radiotherapy Approach for Hepatic Metastases
Plaque radiotherapy for the primary tumor in the eye has been incredibly successful. In order to apply a similar concept to hepatic metastasis, interventional radiologists have begun delivering commercially available yttrium-90-loaded beads to hepatic metastases, in a method called radioembolization.
The first study showing benefit of this approach was a case series published in 2009 (8) that retrospectively studied 11 patients from five centers around the world. The one-year survival for the patients with available follow-up was 80 percent. With such encouraging results, we initiated our own trial with 32 patients, using radioactive microspheres as salvage therapy after chemoembolization and immunoembolization had failed (9). Overall, survival ranged from one month to 29 months, with a median of 10 months. Patients with lower tumor loads at the start of treatment generally had a longer survival rate.
Targeted Therapy for Metastases
Around 95 percent of patients develop metastases to the liver. However, it has not been immediately clear why this organ is so regularly seeded. There are no draining lymph vessels from the eye, indicating that the metastases travel via blood vessels. But it is the capillaries of the lungs, not the liver, that first filter blood from the eyes, and it is not clear why metastasis do not form there first (10).
Molecular analysis of metastatic uveal melanoma provides clues. Some of the most aggressive ocular melanoma tumors over-express receptors whose ligands are highly expressed in the liver. For example, the liver produces a growth factor called hepatocyte growth factor (HGF), which is involved in stimulation of liver progenitor cells and in regeneration of the liver. Many ocular melanoma tumors overexpress the c-MET gene, which encodes the HGF receptor (HGFR). Therefore, liver-secreted factors may draw uveal melanoma metastases to the organ and, as in the case of c-MET, turn on signaling pathways that lead to cancer cell survival and invasive ability. Other receptors that are upregulated in this cancer also have natural ligands that are expressed in the liver, such as the IGF-1 receptor - IGF-1 pairing (11).
A number of clinical trials are underway to break signaling between the liver and the tumor. We are participating in one such trial (12) that combines an antibody-based inhibitor (trametinib) of c-MET (LY2875358) with an inhibitor of the signaling molecules MEK and ERK, which is responsible for the growth in uveal melanoma. In combination, these drugs aim to dampen the signal between the liver and the cancer cells, while also knocking out several downstream signaling molecules. Both MEK and ERK are constitutively activated by mutations in GNAQ and GNA11 genes associated with more aggressive disease (Figure 2). These and other systemically-administered targeted therapies aim to improve outcomes by specifically attacking some of the mutations that make these cancers so aggressive. This includes MEK inhibitor as a single agent or in combination with other signal blockades such as PKC inhibitor, AKT inhibitor and PI3K inhibitor.
Looking Forward
Although we are making progress, all of the approaches tried to date are essentially palliative. Currently, the best chance to cure this fatal disease is early detection before the tumor has spread. It is critical that everyone, especially those with nevi, undergo yearly ocular exams, whether or not a new prescription for glasses is needed. Hopefully, liver-directed therapies, targeted therapy, and immunotherapy will open up new options for patients, as we continue to explore these avenues in treating metastatic uveal melanoma.
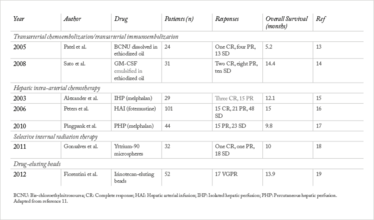
Table 1. Locoregional treatment of hepatic metastasis from uveal melanoma: the evidence base.
Takami Sato is a Professor of Medical Oncology at Jefferson Medical College, of Thomas Jefferson University and Thomas Jefferson University Hospitals in Philadelphia. Carol Shields is a Co-Director of the Wills Eye Hospital Oncology service and is a Professor of Ophthalmology at Thomas Jefferson University.
Acknowledgement: The authors gratefully acknowledge Edyta Zielinska for preparation of this article and editorial assistance.
A Patient’s Story
Carol Mullin describes ocular melanoma diagnosis, and what happened next.
It was March, 2010. I had taken the day off work to take my mother for her eye doctor appointment. Afterwards, she wanted to get her new glasses made, so we went to a company at our local mall that makes glasses in under an hour. While waiting, I noticed that the optometrist's office didn’t look busy. I was due for an eye check-up that summer and I thought, "Maybe I can get it done here, today, instead of waiting." They were able to take me. I wrote a check for $35; she did panoramic pictures of my retinas, and that’s how it got found.
Right away, the optometrist saw that there was a raised area on the retina of my right eye. She pointed to this spot and told me, “It’s raised. I just need somebody to take a look at that”. I was then advised to see a retinal specialist as soon as possible.
The earliest appointment that I could get was three weeks later. When I went, the retinal specialist was quite casual when he started. Once he looked inside my eye, however, his whole demeanor changed. He turned to the technician and said, “Please get Carol Shields on the phone right now”, took me by the hand, and walked me down the hall. He interrupted a staff member who was eating her breakfast and said, “I need two dimensional pictures of her eye right away”. She took pictures of my eyes, and by the time I walked into the consultation room, the picture of the tumor was up on the wall. It was ugly. He didn’t tell me what he thought it was but he did say he was going to send me to an ocular oncologist in Philadelphia. I was very lucky; I had an appointment the very next day.
So the next day, I saw Carol Shields and her husband Jerry. They took more photos and tested my sight; they did all sorts of things. Dr Jerry took my hand and said, “We know what this is and we know exactly what we’re going to recommend to you”. That’s when I found out that I had ocular melanoma. They gave me a booklet, which I’ve kept to this day, and two pages of text detailing the tests that I would have done before surgery, and the tests they’d do regularly after surgery, which they told me to share with my primary care doctor – that was very helpful. I had my surgery two weeks later – they placed a radioplaque on my tumor and took a biopsy at the same time.
Just two weeks after that, at the post-op check-up, they were able to tell me about my risk of metastasis. Dr. Carol told me, “We got the results of your test and we’re going to have the genetic counsellor see you before I do”. The genetic counsellor went over the results the same day – I had the most aggressive form of the tumor: monosomy of my third chromosome and amplification or extra material on chromosome number eight. Basically, I had a 70 to 80 percent chance of metastasis. I have to say that the hardest thing was hearing the genetic result. When I got told the chance of metastasis I physically felt like somebody punched me in the stomach.
But after the genetic results, Dr. Carol and Dr. Jerry immediately referred me to Dr. Sato. Then, the heat treatment and chemotherapy started. The heat treatment around the tumor was to prevent scarring, and I had six Avastin injections on my eye over a two-year period to prevent blood vessels from growing. I didn’t have any metastases at that point, so he treated me for a year with an oral chemotherapeutic agent called Sutent, to help prevent metastasis. I was on that for a year. About six months after I stopped it,the mets showed up. That was in March of 2012.
When I told a physician friend I was going to be on Sutent he said, “Well Carol that’s a really great drug. We use it for GI cancers but you can’t stay on it forever, and when people come off it, the cancer comes back.” In my case it was being used preventatively and I feel like it gave me a year and a half with no mets because I didn’t develop metastasis when I was on the drug, and they really didn’t show up until six months after I was off it. I wasn’t that surprised when they did turn up. To me it was not a question of if I would get mets, it was more of a question of when. The cancer metastasized to the liver.
Dr. Sato is my oncologist, but he works closely with two interventional radiologists, Dr. Eschelman, and Dr. Gonsalves, who do the liver-directed treatments. Initially I received four infusions of Yervoy over a four month period. This was followed by eight immunoembolization treatments. and now radioembolization therapy with little radioactive beads.
I’m still hopeful. I just had scans done yesterday and I see Dr. Sato next week. I’m hoping that the radioembolization has shrunk the tumors, but I’ll find out if that’s the case at the appointment. The immunoembolization was great; it didn’t shrink the tumors, but it gave me stable disease – it bought me time. Most of the time I feel good. I don’t sit and worry about how many years I have.
- C.L. Shields, S. Kaliki, M. Furuta, et al., “Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8033 cases”, Retina, 32, 1363–1372 (2012).
- C.L. Shields, M. Furuta, A Thangappan, et al., “Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes”, Arch. Ophthalmol., 127, 989–998, (2009).
- C.L. Shields, S. Kaliki, M. Furuta, et al., “Diffuse versus non-diffuse small (< 3 millimeters thickness) choroidal melanoma: Comparative analysis in 1751 cases. The 2012 F. Phinizy Calhoun Lecture 2012”, Retina, 33, 1763–1776 (2013).
- J.W. Harbour, “A prognostic test to predict the risk of metastasis in uveal melanoma based on a 15-gene expression profile”, Methods Mol. Biol., 1102, 427–440 (2014). doi: 10.1007/978-1-62703-727-3_22.
- T. Sato, D.J. Eschelman, C.F. Gonsalves, et al., “Immunoembolization of malignant liver tumors, including uveal melanoma, using granulocyte-macrophage colony-stimulating factor”, J. Clin. Oncol., 26, 5436–5442 (2008).
- D.J. Eschelman, C.F. Gonsalves, M. Terai, et al., “The results of a randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) in uveal melanoma patients with hepatic metastasis”, J. Clin. Oncol., 29:544s (2011).
- R.D. Carvajal, J.A. Sosman, F. Quevedo., et al., “Phase II study of selumetinib (sel) versus temozolomide (TMZ) in gnaq/Gna11 (Gq/11) mutant (mut) uveal melanoma (UM).”, J. Clin. Oncol., 31, suppl, abstr CRA9003, (2013).
- A.S. Kennedy, C. Nutting, T. Jakobs, et al., “A first report of radioembolization for hepatic metastases from ocular melanoma”, Cancer Invest., 27, 682–690 (2009).
- C.F. Gonsalves, D.J. Eschelman, K.L. Sullivan, et al., “Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience”, AJR Am. J. Roentgenol, 196, 468–473, (2011).
- S. Bakalian, J.C. Marshall, P. Logan, et al., “Molecular Pathways Mediating Liver Metastasis in Patients with Uveal Melanoma”, Clin. Cancer Res., 14, 951–956 (2008).
- .A.P. Asija, M. Yoshida, T. Sato, “Treatment strategies for clinically detectable metastatic uveal melanoma”, In: Ocular Melanoma: Advances in Diagnostic and Therapeutic Strategies. T.G. Murray, H.C. Boldt (Eds). eISBN 978-1-78084-426-8. Future Medicine, London, UK. Pages 169–191 (2014). In Press.
- National Institutes of Health (NIH). ClinicalTrials.gov. clinicaltrials.gov/ct2/show/NCT01287546. Updated December 10, 2013. Accessed April 25, 2014.
- K. Patel, K. Sullivan, D. Berd, et al., “Chemoembolization of the hepatic artery with BCNU for metastatic uveal melanoma: results of a Phase II study”, Melanoma Res., 15, 297–304 (2005).
- T. Sato, D.J. Eschelman, C.F. Gonsalves, et al., “Immunoembolization of malignant

A native of Kyushu, the third largest island of Japan, Takami Sato is one of the world’s leading physicians in the field of metastatic uveal melanoma therapeutics. Now based in the US at Philadelphia’s Jefferson University, Sato is the Director of their Metastatic Uveal Melanoma Program.
Carol Shields is Chief of the Ocular Oncology Service at Wills Eye Hospital and Professor of Ophthalmology at Thomas Jefferson University in Philadelphia, USA