Not the Usual Suspects
The secret to managing irregular astigmatism during cataract surgery? Tackling the problem at its source.
At a Glance
- A lot of talk about astigmatism and intraoperative aberrometry is made on the assumption that the cornea is normal and regular. But what if it isn’t?
- Biometry becomes far more difficult, as does IOL power calculation. Get it wrong, and there’s grief all round.
- Understanding the causes of irregular astigmatism – and how do you deal with them – is key.
- Here, I offer general principles of how to manage these ‘irregulars’ and give a few case examples of my own.
When people talk about astigmatism and intraoperative aberrometry, the conversation is often made with the assumption that the cornea is normal and regular. When it isn’t normal and regular… well, you have a problem. I have been dealing with some pretty difficult corneas for a quarter of a century now, so let me share with you what I have learned over the years.
Irregular astigmatism in patients presenting for cataract surgery (or refractive lens exchange; RLE) can have consequences: poor vision. For the surgeon, this is partly because the biometry can be challenging, which makes accurate IOL calculations difficult. Any error results in a refractive miss, causing both the patient and surgeon grief.
To deal with irregular astigmatism, we first have to understand its cause.
Why corneas become irregular
Let’s look at the cornea systematically from front to back. There are five layers and three of them are responsible for irregular astigmatism (Figure 1). The tear film sits atop the cornea, and needs to be regular in its consistency to provide regularity of the corneal surface. The epithelium translates to the tear film and, if that is irregular then, once again, we have irregular astigmatism. You cannot forget the subepithelial area; there are conditions that affect this region and they also translate right across to the front surface of the cornea. And then there is the stroma; if it is scarred, ectatic or abnormal in in terms of shape that may also cause irregular astigmatism.
Dry eye
Dry eye is probably the single biggest cause of irregular astigmatism. It’s something that cannot be dismissed – and, in this age of refractive lens exchange and refractive cataract surgery, we are appropriately giving this topic a great deal of attention. We all know the causes of dry eye: underproductive, evaporative and inflammatory, but remember, an irregular tear film can exist in asymptomatic patients.
Tear osmolarity can be used as a measure of dry eye, and Epitropoulos et al. (1) showed that patients with hyperosmolar tear film occasionally display great variation in terms of their keratometric cylinder (Figure 2) – plus, their IOL calculations varied by 0.5 D in 10 percent of cases. Dry eye really is not a trivial disorder, and it certainly does not have a trivial impact on IOL calculations.
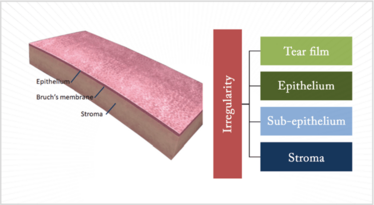
Figure 1. Disorders of the tear film, or any one of three corneal layers – the epithelium, sub-epithelium or the stroma – can all cause irregular astigmatism.
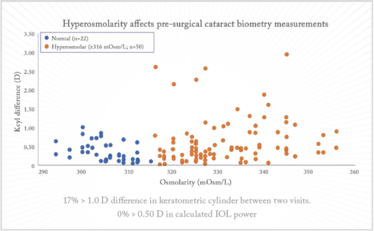
Figure 2. Keratometry cylinder (1). Seventeen percent of hyperosmolar eyes had at least 1.00 D of change in keratometric cylinder values between two visits. The hyperosmolar group demonstrates a wider variation in keratometric cylinder between visits relative to the normal group (p=0.013).
Epithelium-induced irregularastigmatism
Figure 3 shows a case of anterior basement membrane dystrophy – also known as epithelial base membrane dystrophy, Cogan’s dystrophy, map-dot-fingerprint dystrophy... Whatever you call it, the irregularity caused in the cornea makes accurate calculation very difficult.
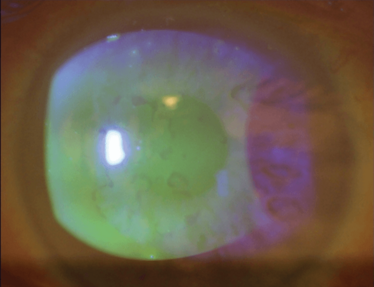
Figure 3. Anterior basement membrane dystrophy – also known as epithelial basement membrane dystrophy, Cogan’s dystrophy or Map-dot-fingerprint dystrophy – results in irregular astigmatism.
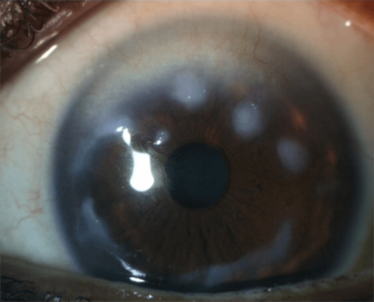
Figure 4. An example of a sub-epithelial disorder, Salzmann’s nodular degeneration, that causes irregular astigmatism. Other disorders include scarring of Bowman’s layer and Reiss-Bücklers corneal dystrophy.
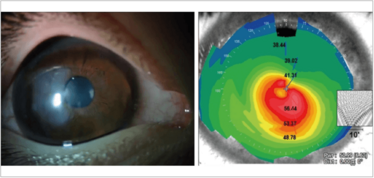
Figure 5. An eye with keratoglobus, scarring and an extremely thin cornea (left) – and a very unusual topographic map (right).
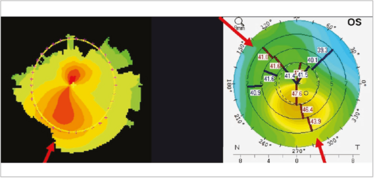
Figure 6. Irregular astigmatism: corneal shape. Asymmetric (left) and non-orthogonal astigmatism (right).
Irregular astigmatism arising from the sub-epithelium
Salzmann’s nodular degeneration (Figure 4) can also cause problems. Even if the degenerative nodule is peripheral, it can influence central keratometry and regularity. You may be able to risk performing a cataract operation on this patient – but, if the nodules progress and eventually need removal, you have got a whole new keratometry to deal with; each case needs to be carefully considered.
Stromal irregular astigmatism
Both scarring and ectatic disorders can cause irregular corneal astigmatism from the stroma. The patient in Figure 5 actually has a combination of both: keratoglobus with scarring and an extremely thin cornea, which has resulted in a very unusual topographic map. Such patients are not easy to deal with.
Corneal shape changes can commonly cause irregular astigmatism. They often present as asymmetric bow ties; if they’re orthogonal, then they are on the correct axis, the cornea is just steeper inferiorly than above (Figure 6) – and that’s not bad, because the overall astigmatism is fine – it’s when they’re non-orthogonal that it’s a problem. If you’re going to use a toric lens in a non-orthogonal astigmatic patient, then the biggest question is: where do I orientate the implant? I shall address this later in this article.
Diagnostics of Irregular Astigmatism
We are doctors – and one of the most fundamental aspects of the profession is evaluating patents clinically. A comprehensive clinical evaluation is vital in diagnosing irregular astigmatism, but we do also have a number of diagnostic instruments available to help us. And they can also be used to monitor disease progression (and improvement) of patients. When we look at a patient, going from front to back; first, we look at the tear film, then look at the shape, clarity and surface of the cornea to see if there is anything that might influence things. Next comes a fluorescein stain to check lid action and the distribution of the fluorescein dye. If patients are not closing their eyelids properly and have exposure, then they may need to be seen by an oculoplastic surgeon before proceeding to cataract surgery.
The RLE-requesting web developer with terrible tear films
This video depicts the eye of a patient who came to see me for refractive lens exchange. He was adamant that he wanted trifocal lenses implanted; however, after examination, I had to tell him, the bad news “Your tear film is terrible and you have got a whole load of other problems that need attention (punctate epithelial keratopathy and anterior basement membrane dystrophy)!” He even had an acute chalazion in his fellow eye! My patient was certainly not ready for refractive lens exchange.
His tear film was streaky, so no surprise by his report of fluctuating vision – which he thought it was from his cataracts. There was no way that I was going to operate on him until I fixed his ocular surface, a process that took about three months, whereupon we proceeded with the RLE, successfully implanting trifocal IOLs.
Video available at top.txp.to/issues/0518/501.
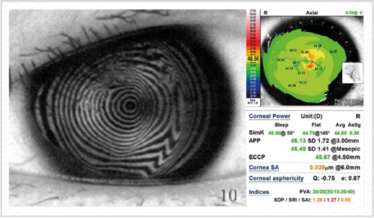
Figure 7. Placido disk-based diagnostic systems are a great way of evaluating the extent of corneal irregularity.

Figure 8. Anterior basement membrane dystrophy is commonly associated with meibomian gland dysfunction and may be related to inflammation. The pathology is a duplication of the basement membrane. The irregularity it imparts onto the cornea is best removed either by epithelial cell debridement (see the video at top.txp.to/issues/0518/501) or phototherapeutic keratectomy.
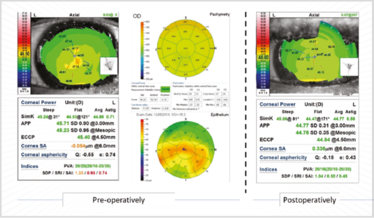
Figure 9. The difference epithelial debridement has on corneas with anterior basement membrane dystrophy.
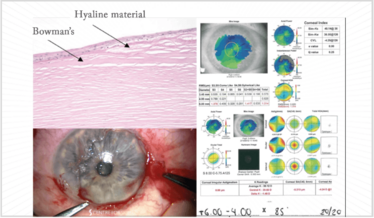
Figure 10. Clockwise: the corneal pathology of Salzmann’s nodular degeneration (SND), keratometry, axial power and HOA maps, and a still from a video of a superficial keratectomy procedure performed on the most extensive SND patient I have ever seen. The video is available at: top.txp.to/issues/0518/501.
What tools do we have at our disposal, if we suspect there is a problem and want to know the magnitude of that problem?
First, you need a Placido-based reflective topographic system (Figure 7), and there are some great markers devised by Steve Klyce that can be used to flag irregular corneas: the Surface Regularity Index (SRI) is used a lot; the Standard Deviation of (corneal) Power is also a good one. In Figure 7, the SRI value is colored in red – and that indicates there is something going on with this patient’s eye – anterior basement membrane dystrophy in this case.
Some Placido systems are combined with aberrometry systems (such as the Nidek OPD 3, Tracey Technologies’ iTrace and Topcon’s KR1W). They all work in slightly different ways, but all are excellent at providing truly useful information about the surface and overall aberrometry, and by subtracting corneal-derived aberrations from the overall aberrations, internal aberrations can be calculated. Three-dimensional diagnostic instruments (for example, the Orbscan [B+L] with its scanning slit approach or the Scheimpflug-based systems: Oculus’ Pentacam, Ziemer’s Galilei or Visionix’s VX120 family) offer shape analysis – elevation, posterior cornea and corneal thickness, which is really useful information when it comes to dealing with the corneal shape. Anterior segment OCT is now coming into vogue and I suspect will displace some of the current “gold standard” devices being used.
Management strategies
The principles of management are simple: eliminate and reduce corneal irregularity by addressing the source of the problem by either medical therapy (for example, when the cause is dry eye) or surgical intervention. Follow-up is important; the cornea needs to be evaluated to see how the patient’s eye is progressing. Has the surface got smoother; has the tear film improved? Has the surface stabilized?
If a patient presents with dry eye, evaluate the cause and treat it. Once the tear film is stable, biometry and IOL calculations can be performed with some confidence. The key is to proceed from stable foundations, rather than highly fluctuating ones. But what about the aforementioned corneal disorders?
Anterior Basement Membrane Dystrophy
Anterior basement membrane dystrophy results from a duplication of basement membrane, which causes an irregularity you can see on the fundus retroillumination picture (Figure 8). Typically, epithelial debridement (even at the slit lamp) followed by a bandage contact lens is sufficient in such cases – occasionally we’ll add photo-therapeutic keratectomy (PTK) – 5–10 µm laser ablation in PTK mode – to promote good healing and adherence.
The patient in Figure 8 had quite a high SRI; the OCT-based epithelial maps show the difference between corneal irregularity before and after epithelial debridement (Figure 9). His keratometry changed from 45.71 D to 44.77 D – just over 1 D change – and his irregularity index went from orange and red values to green ones. The cornea became more regular with better potential vision. Six weeks later, these values remained stable, which meant we felt comfortable enough to perform ocular biometry and IOL calculations.
Salzmann’s Nodular degeneration
A patient presented for a second opinion to the Centre for Sight for RLE with a refractive error (+8.00 D). He became worried, when he realized his original surgeon was a little hesitant. When I examined him, I saw marked 360° nodular degeneration and an irregular cornea. Salzmann’s Nodular degeneration is caused by hyaline that sprouts out from breaks in Bowman’s layer and goes on to the surface (Figure 10). In terms of removal typically the edge can be found and elevated and removed as in the image.
Postoperatively, he had a smooth regular cornea that was steeper; his refraction changed considerably and Figure 11 shows his keratometry: he was going to have a 28.5 D lens placed in his left eye (with flat Ks), but after the procedure he only needed a 20 D lens – an 8 D difference (there was a 4.5 D difference in the right eye).

Figure 11. Pre- and post-op keratometry of a patient with Salzmann’s Nodular Degeneration who underwent superficial keratectomy.
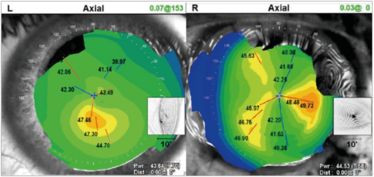
Figure 12. An illustration of a patient with non-orthogonal astigmatism.
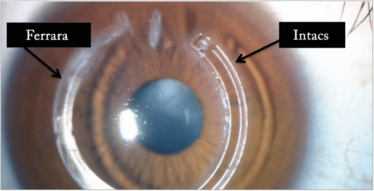
Figure 13. A Ferrara and an Intacs ring (CorneaGen) in the same eye of a patient.
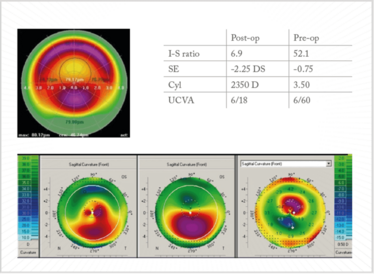
Figure 14. CXL and topo-guided PRK. Case courtesy of Arthur Cummings.
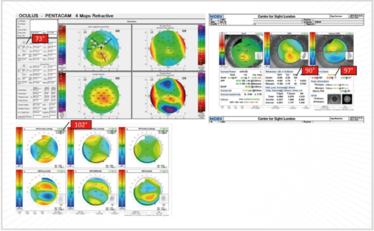
Figure 15. Non-orthogonal astigmatism: which axis to use? The Pentacam says 73°; OPD indicates (by refraction) 90° and (topography) 97°, and the corneal-derived Z2-derived aberrometry polynomial for astigmatism (Z2±2 S2±2) indicates 102°!
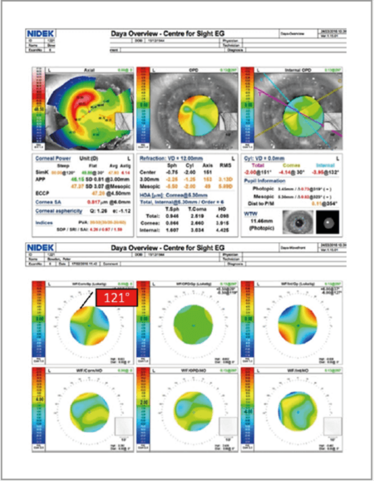
Figure 16. Surface maps and aberrometry of a patient with a prior corneal transplant.
Non-orthogonal astigmatism
What causes non-orthogonal astigmatism (Figure 12)? Ectatic disorders like keratoconus mostly, but it can also occur in post-corneal graft patients. So how does one deal with those patients who have got corneal ectasia or keratoconus?
I always try and find out from the optometrist what the BSCVA was before the cataract. If it was 6/12 or better I’d probably go ahead and do a lens calculation and put a toric lens in. If it was less than 6/12, then I’d have to try and find a way to regularize the corneal shape either by use of intracorneal rings (Figure 13) or corneal cross-linking (CXL) with topo-guided PRK. The last resort would be corneal transplantation.
Arthur Cummings of the Wellington Eye Clinic, in Dublin, Ireland, shared the details of another case with me – a patient who underwent CXL and topo-guided PRK (Figure 14). He had a pre-op I-S ratio of 52.1 – but, after the procedure, the ratio had reduced to 6.9; similarly, UVCA was 6/60 before surgery, and 6/18 afterward, and – importantly – the patient was now ready for ocular biometry, lens calculation, and a toric IOL implantation.
Which axis do you use non-orthogonal astigmatism?
In Figure 15, the Pentacam shows 73° simulated steepening; the OPD (on refraction) shows in the 90° axis, and on topography, 97°. Which axis does one use? Well, to confound this further, I prefer to use the corneal-derived Z2-derived aberrometry polynomial for astigmatism. We’ve been doing this for quite some time now, and we’ve found that it very accurately averages out the actual axis. In this particular case, 102° is the axis that needs to be used.
These diagnostic devices can also provide a good indication of orientation of the toric lens postoperatively without dilation. Figure 16 shows the surface maps and aberrometry of a patient who had a corneal transplant before I had implanted a toric IOL. Before the procedure, I had intended to place the lens at a 121° axis – but it turned out it was actually placed at 127°, giving the patient a residual refraction of 2 D. I always aim for emmetropia – but it’s not always possible in these types of patients – and so they need to be counseled in advance about this possibility.
The bottom line
As noted, the principles of managing irregular astigmatism cataract surgery are actually pretty simple: find the source of the problem, address it as best as you can, then only proceed to ocular biometry, IOL calculations, and surgery when the astigmatism is compatible with potentially good vision. If you can follow those rules, then you needn’t fear irregular astigmatism in cataract surgery: you can embrace it instead!
Sheraz Daya is the Medical Director of the Centre for Sight, East Grinstead, Sussex UK, is a member of The Ophthalmologist’s Power List 2018 Top 100, is internationally renowned for his work in laser eye surgery, lens replacement, stem cell transplantation, corneal transplantation and refractive cataract surgery, and was recently awarded the Fyodorov Medal by the Hellenic Society of Intraocular Implant and Refractive Surgery.
He reports that he has consulted for Bausch + Lomb, Carl Zeiss Meditec, Excellens, LinCor Biosciences, Medicem, Nidek, STAAR Surgical, Scope Pharmaceuticals, and Rayner, is an equity owner in Excellens, PRN and Strathspey Crown, has received grant support from Johnson and Johnson Vision, and lecture fees from Bausch + Lomb and Nidek.
- AT Epitropoulos et al., “Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning”, J Cataract Refract Surg, 41, 1672–1677 (2015). PMID: 26432124.
Daya is Medical Director of the Centre for Sight, East Grinstead, UK. He has been an innovator and leader in refractive and cataract surgery. He has both led and been involved in classification systems and consensus panels and avidly supports good use of language that facilitates communication amongst stakeholders.