Head in the Clouds
Organ-to-cloud technology is here – and it has its sights set on glaucoma. Offering a stream of objective, uncompromised data, it could revolutionize the glaucoma space… But for it to work, we need to rethink the way we assess therapy effectiveness in our current treatment paradigm.
Ariel Cao | | Longer Read
When was the last time you bought a CD? I’m guessing it was a long time ago. Nowadays, you probably pay nine dollars a month for a music streaming service that lets you listen to whatever song you like, whenever you like, on whatever device you like – amazingly, with minimum fuss.
Given the choice, I doubt you would go back to CDs. Why? Because streaming makes sense. We live in an era of constant connection, where we can access the information we need anytime, from anywhere. Real-time event updates mean we can predict and plan for change. Yet, somehow, this does not translate to the medical field. An ophthalmologist treating a progressive disease like glaucoma can’t be anything but reactive; critical data on a patient’s disease state is only collected during sporadic office visits, yielding only a few single data points to interpret past trends and assess long-term risk – often with harmful results.
Patients are paying for therapies without knowing whether they are actually having the desired impact on their intraocular pressure (IOP) – and some are going blind as a result. Under our current treatment system, if a therapy doesn’t work, the patient doesn’t pay. But who benefits from this model? Not the patients – they don’t see an improvement in their condition. And it’s not the drug companies – they are being penalized by the reimbursement system for ineffective therapies. It is clear to me that it’s time for a new treatment paradigm – one that proves outcome with evidence. But how do you know if a therapy actually works? Easy – you follow the data. And that’s where we come in.
We are Injectsense – a sensor-enabled digital health company – and we have developed an organ-to-cloud data connection that enables clinicians to assess glaucoma therapy effectiveness at any time. A self-anchoring system is inserted into the vitreous base – the best place to measure pressure on the optic nerve – where it safely and autonomously collects pressure readings. This aspect is important: patient intervention has been shown to be the least effective pathway to improved glaucoma outcomes (1, 2). The device automatically syncs to the cloud every week, offering physicians unprecedented visibility into the patient’s IOP profile and into previously unobserved changes that may affect glaucoma progression.
Once the device is implanted in the eye, it lies dormant until it takes an IOP reading. It is not affected by atmospheric pressure – the patient could go to the bottom of the ocean or the top of Mount Everest and the device will continue to work. Once a week, the data is sent to the cloud for the physician to access. So we have the data – what happens next?
Let’s go back to CDs and music streaming. CDs contain data. The servers of the music streaming service contain data. What makes music streaming so special is how that data can be presented and used. A music streaming service doesn’t just give you access to the CDs (or tapes or vinyl records) you used to own – or the music you want to own – it opens up a whole world of music. It makes recommendations, enables you to share songs or entire playlists with friends on the other side of the world, it allows you to discover something new. Music streaming has reinvented the way we consume music – and it has all but killed the traditional music store...
What if we started to treat glaucoma management like a streaming service? What if a patient doesn’t buy a therapy, but subscribes instead? They could choose a treatment that suits their specific medical needs and budget, and if it doesn’t work – and with our device, they will actually be able to tell – they try something else. The premium IOL market has proven that patients are willing to pay out of pocket for improved outcomes – and we are in the position to provide them with evidence of efficacy. A product like ours has the potential to guide drug and device development based on efficacy; no more wasted dollars for the industry and no more pointless treatments for patients.
We have already had success with the device, having recently completed our first in-vivo animal study. We safely collected a week’s worth of readings – in agreement with tonometry measurements –and reported no device-related adverse events. If everything goes well, we will begin our first controlled human study at the end of 2020. From there, the sky’s the limit.

The origin story
Eight years ago, my parents were affected by glaucoma. My mother was prescribed topical drops but they stung her eyes, so she stopped taking them, and even omitted to tell the physician about her situation. As a result she lost her eyesight in one eye. Around the same time, Enrique Malaret, our COO and co-founder of Injectsense, had also come up against the disease. We decided we would do what we could to help. The challenge spoke to us as individuals, as much as engineers. How do we determine whether a treatment actually works? Our solution was a continuous monitoring device, taking the form of an injectable ultra-miniature sensor coupled with a secure digital health platform. We spent the next three years fleshing out the concept and securing funding – once we had enough, we began to scale.
As you can imagine, creating an organ-to-cloud system is not easy. It takes time to develop a premium product, especially one that draws from several domains – such as medicine, semiconductors, fluid interactions, and electronics. Putting together a team that could connect these disciplines without leaving gaps was critical. We made sure to pick people with overlapping skills, and ensured we worked as one collective entity. The team now stands at 10-plus full time employees, alongside a pool of contractors. We choose to keep our team small because, the more people you bring in, the less efficient you become.
Staying small keeps us agile. It has also makes it easier for everybody to know their roles, which is important, as two-thirds of the start-ups fail because of internal conflict. We made a policy of looking for people who are hands-on, team players, with proven multidisciplinary expertise, and who are motivated well beyond simple monetary payback. We have stuck to it. Together, we have built a team that has developed a solution to be proud of.
So how does it work? First, the implant is delivered in-office via an injection, leaving a self-anchoring sensor in the vitreous base. The implant begins to measure IOP at a series of times predetermined by the physician. Once a week, the patient is reminded to upload the data by putting on a pair of glasses – this notification will come from an app on the patient’s phone. Uploading takes less than a second. At this point, the data is sent to the cloud, where it is ready to be mined by our software. We start by removing data that has no purpose (such as IOP increases caused by blinking) and instead, look for information that is actionable, which is to say, pressure readings that exceed a certain value over a given period of time.
The idea is to build a histogram representative of the pressure exposure. The physician can then access the data and see which patients, if any, have exceeded their set target. We also provide physicians with the option to decide how often patients are sampled – for example, once every ten minutes or once an hour. The next time the patient uploads data via the glasses to the cloud, the new sequence will be automatically downloaded.
The whole system requires very little effort from the patient. Minimizing human error was one of our core aims, as patients are often the weakest link in the treatment process. Every physician can provide an example of a case where patient non-compliance has resulted in less than ideal outcomes. This issue is particularly common in glaucoma, where patients do not necessarily see an immediate benefit in taking their medication but may experience side effects. The patients also may not see the urgency, as they don’t experience pain, and the disease typically progresses very slowly.
Imagine how many people’s vision could be preserved if there was a way of showing them how their treatment program was working. Crucially, the system also requires little physician time. By 2025, 50 percent of the ophthalmic workforce will have retired – but the number of students entering residency is dropping off. Devices like ours will play an important role in tackling the increasing treatment burden, made worse by an aging population.
Our device may also have significant potential in terms of patient responsibility. Too many ophthalmologists are exposed to malpractice claims. Wouldn’t it be helpful if they could arm themselves with the evidence that it was, in fact, the patient who hasn’t complied with recommendations? That’s not to say we are going to act as a mediator between insurers and patients – rather, our data can empower patients to take their medication as prescribed, and everyone – physicians included – can benefit from the results.

From left to right, pictured holding devices: Ernesto Collazo, MD, FAAO, and member of Injectsense Medical Advisory Board; Enrique Malaret, Chief Operating Officer and co-founder; Arthur Sit, MD, FAAO, and member of Injectsense Medical Advisory Board; Ariel Cao, Chief Executive Officer and co-founder.
Tackling compliance with data
Our current gold standard method for measuring intraocular pressure – Goldmann applanation tonometry – is plagued by fudge factors. Although it’s considered the “gold standard” with broad acceptance, for some cases it may seem more like a “random number generator.” Due to the lack of consistent calibration, operator error and variability of measurement conditions, the value may not be 100 percent trustworthy. One day it’s this, the other it’s that and nobody knows why.
Ophthalmologists use tonometry as a relative measurement – but I believe we can do better. All it takes is a shift in mindset. We are lucky enough to have some incredibly influential figures on our advisory board – Ike Ahmed, Myron Yanoff, Arthur Sit, Richard Lindstrom – but what is reassuring is that once we start talking to them about the device is that within 10 minutes they play back the benefits that they perceive from their own experience. It’s what they’ve been waiting for – to be more effective in their therapy management. Now they can personalize therapy for each patient and transform the relationship to contain the disease progression more effectively. After all, only 30 percent of glaucoma patients are compliant with medication use. Our device may help to gain an additional 5 percent, 10 percent, maybe even more, of those patients, and improve outcomes with a cost-effective drug regimen, by just taking the drug.
The device may also offer unparalleled insight into drug efficacy – separating the losers from the winners. Companies who sell drugs that don’t work will have no place to hide. And though that sounds damning, it will ultimately be a positive thing. Instead of spending money developing drugs that don’t work, we could funnel funds into ones that do.
A Physician's Perspective
Ernesto L. Collazo, ophthalmologist
On the subject of glaucoma, I often explain how difficult it is for ophthalmologists to manage a disease where our goal is to control IOP but we only get to measure that pressure at three or four points during the year. None of my internal medicine colleagues would ever try to manage the insulin dosage in a diabetic patient with only four measurements of blood sugar in a year, but we are forced to manage glaucoma that way.
It was Enrique who suggested that some sort of miniature implantable device could potentially give us the ability to measure IOP throughout the day. I mentioned that the best place to implant the device would be the Pars Plana – the basic idea for what Injectsense is developing today was born at that moment. Once Enrique and Ariel founded Injectsense, they contacted me to become their first medical advisor and I recruited some of the other current members of the medical advisory board. The rest is history.
Monetizing the data
People ask how physicians can use the system to operate within a viable business model. Simple – they charge for the data plan. The true value of this system comes from data-driven therapy management. For the first time, physicians will be able to check a patient’s IOP outside of clinic hours. Whenever a patient has repeat IOP spikes throughout different periods during the day, the system will alert the doctor. At the end of the week, they can download that data and decide on the best course of action for that particular patient. The benefits of this cloud-based system are two-fold. One: it is considerably cheaper – and easier – to move data than to move the person (to the doctor’s office). Two: it eliminates unnecessary visits to the doctor’s office, saving patients’ – and physicians’ – time and effort. When they do visit the clinic, it is for a justifiable intervention.
The idea is that providers receive a monthly fee for monitoring under a CMS reimbursement code, and more codes are being introduced every year. For some, this may seem like an unworkable business model – but it isn’t. Medtronic, the world’s largest medical technology company, began offering embedded advanced data analytics in its clinical monitoring software to enable cardiologists to identify and remotely monitor patients, allowing them to act faster on potential health risks. They understood what so many didn’t: that the future of healthcare is data-driven – and we see it too.
We cannot claim to cure glaucoma but we can help patients manage it, by providing ophthalmologists with an objective, unambiguous body of clinically actionable data. The database could also become invaluable to research centers and public health organizations.
Digitizing the future of healthcare
Glaucoma is just the start. Sensor systems are renowned for both their stability – and their versatility. The device has the potential to be used in any field that measures fluid, from urology to neurology. Say a patient had brain cancer, we could put a reservoir next to the device to facilitate drug delivery. We could also replace the pressure sensor with an electrode to stimulate the nerve, for use in neuromonitoring and neurostimulation applications.
Our device is not a one-trick pony, it is a complete digital health platform. It may be diagnostic today, but could easily be extended to a therapeutic platform tomorrow. Even before initiating human trials, we plan to apply for theFDA’s Breakthrough Device Designation that enables a waiver of the economic outcome study in order to set an early reimbursement value immediately. We fully meet all the criteria for this designation.
The mindset around medicine is changing. We, as members of the industry, have a responsibility to offer patients a better outcome, a better quality of service and a better quality of care. Glaucoma patients deserve a return on health – and we want to help make that happen. Our employees have invested years on this enterprise. When all is said and done, we feel that we have found the simplest, most elegant way of monitoring IOP, with tremendous flexibility to fit unanticipated needs.
Since our approach is inspired by intravitreal injections – now performed yearly in their millions–, we have a path of least resistance with a simple injection for safe delivery of an IOP monitoring device.
Inside the Injectsense
Jose Padovani, Senior R&D Engineer at Injectsense, explains how the device works
Our design is based on proven sensor technology. The device is less than two cubic millimeters in volume, delivered by injection, and self-anchoring. The silicon sensor is self-packaged, meaning it requires no further encapsulation. As it relies on semiconductor manufacturing techniques, volume production can scale into millions of units per year.
Each wafer has 14,000-plus devices, no different from cell phone components. Its size is one of its greatest advantages: the implant is so small the animals were unaware of its presence. With no intervention required for data collection, and using wireless data downloads weekly or daily, we were able to obtain highly accurate data over an extended period of time in a consistent manner. Data with a unique patient/animal ID was encrypted and stored in a cloud database.
The device itself is built with the same technology that lets you buy groceries with Apple Pay. A tiny antenna inside the implant is coupled with an external antenna, paired to a wearable reader – a mechanism we use to remotely deliver power to the device and extract data. It is ultra-low power, operating at a nanowatt power level, which makes it harmless to the body.
Outside of its internal electronics, it is polymer-free, biocompatible (a well understood foreign body response) and fully hermetic, so that it can operate for decades inside the body. A physician can implant the device and will only need to remove it – a simple surgical explant procedure – in the event of a chronic infection. The design allows the device to be delivered in-office, avoiding the cost of an operating room procedure.
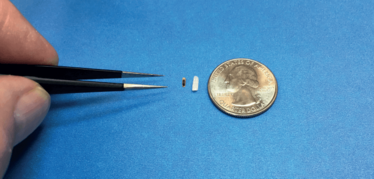
The prototype of Injectsense IOP sensor used in animal testing, next to a grain of rice and a quarter.
- Glaucoma Research Foundation (2017). Accessed: 02.01.2020. Available at: https://bit.ly/2HzYSzq.
- Healio Ocular Surgery News (2002). Accessed: 17.02.2020. Available at: bit.ly/2HwU8dE