The Chronic
IRISS registry 24-month data shows when it comes to DME, there’s chronic and then there’s chronic – and treatment outcomes differ accordingly
An argument rattles on through the ages: what’s the value of a clinical trial, if real-world results don’t match up? There are many reasons for the disparity – the two main ones being i) careful trial patient selection that doesn’t match the characteristics of real-world patients, and ii) closer supervision and dose adherence in the trial than in practice. But when it comes to long-acting implanted formulations of drugs, the second point is moot; patients should continue to receive therapeutic doses of the drug for the lifetime of the implant. And what if the population in the real world was very different to the clinical trial? It was against this background that the five-year, international, multicenter prospective open-label Iluvien Registry Safety Study (IRISS) (1) was performed in 593 eyes (from 563 patients with a [chronic] diagnosis of diabetic macular edema [DME], mean age 67.5 ± 10.7 years, 56.3 percent male) to answer the question: does the sustained-release fluocinolone acetonide 190 µg intravitreal implant work to treat DME in the real world – and for how long?
When it comes to treating patients with DME and using steroids over an extended period of time, there are two main safety signals that ophthalmologists look out for: raised intraocular pressure (IOP) and the development of cataract. At baseline, 82.6 percent of patients were already pseudophakic, and of the 97 patients who were phakic at baseline, 24 received concurrent cataract extraction with Iluvien injection. Mean DME duration at baseline was 4.49 ± 3.3 years, and mean IOP was 15.6 ± 3.86 years (and 5.2 percent of patients had IOP > 21 mmHg at baseline – which would have disqualified them from participating in the Phase III FAME trial (2) that formed the basis for Iluvien’s registration approvals).
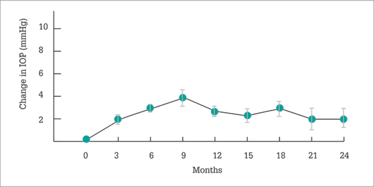
Figure 1. Mean change in IOP. Error bars represent the standard error of the mean.
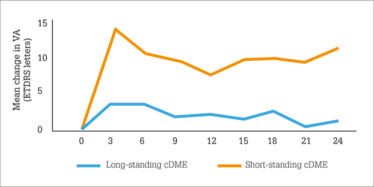
Figure 2. Earlier Iluvien use (i.e. in short-standing chronic DME [cDME]) was associated with better visual acuity outcomes than use in patients with long-standing DME.
Two years after Iluvien was administered, what did the IRISS investigators find? A small (1.9 mmHg) increase in mean IOP (Figure 1) – much like FAME, but despite the wider patient population treated – with no clinically significant change in cup-to-disc ratios being observed. Visual acuity stability or improvement after Iluvien administration was rapid, and sustained over two years and, importantly in patients with chronic DME, earlier Iluvien use was associated with better VA outcomes with fewer IOP-related events (Figure 2). This finding may seem counter-intuitive when faced with the FAME trial, in which patients with chronic DME did better on Iluvien than those with non-chronic DME; however, in IRISS, the patient populations were very different – all patients had chronic DME, and the cutoff between long-standing and short-standing DME (Figure 2) was three years. Longer chronic disease, unsurprisingly, results in poorer outcomes. Finally, no statistical difference (p=0.584) was noted in vision improvement between phakic and pseudophakic eyes.
- U. Chakravarthy et al., “ILUVIEN® (190 micrograms fluocinolone acetonide) real-life safety and effectiveness following usage in three European countries – results from the 2016 extract of data from the ILUVIEN Registry Safety Study (IRISS)”, presented in Free paper session 2: Vascular diseases and diabetic retinopathy I, 17th EURETINA Congress, Barcelona, 7th September 2017.
- PA Campochiaro et al., “Long-term benefit of sustained delivery fluocinolone acetonide vitreous inserts for diabetic macular edema”, Ophthalmol, 118, 626–635 (2011). PMID: 21459216.
I spent seven years as a medical writer, writing primary and review manuscripts, congress presentations and marketing materials for numerous – and mostly German – pharmaceutical companies. Prior to my adventures in medical communications, I was a Wellcome Trust PhD student at the University of Edinburgh.