Taking the Heat out of Retinal Laser Therapy
There’s currently an unmet need for effective interventions in early-stage retinal degenerative diseases – before vision is lost. Can three-nanosecond pulse laser therapy meet that need?
At a Glance
- There’s very little that can currently be done to treat early-stage AMD, short of
offering nutritional supplements - Three-nanosecond laser therapy has shown potential in delivering effective treatment of early-stage retinal disease, safely
- The (non-thermal) laser energy selectively targets RPE cells, ablating the affected area, and allowing healthy RPE cells to migrate in
- This technology could be an effective intervention in early-stage AMD –before significant vision loss has occurred
Typically, laser-based treatment of retinal diseases uses conventional retinal laser photocoagulation with pulse durations of between 100 and 200 milliseconds, with an “endpoint” of tissue whitening. In the case of diabetic retinopathy, retinal laser photocoagulation has been considered standard of care for decades. The resulting lesions, however, routinely affect the retinal pigment epithelium (RPE) and the outer retina including the photoreceptors and the inner nuclear layer. These lesions cause permanent scarring which creates central visual scotomas, reducing patients’ visual function and adversely affecting color and night vision (1,2). Additionally, current retinal laser photocoagulation can be associated with glare and occasional pain.
Further, reports from the Diabetic Retinopathy Clinical Research Network (DRCR.net) have highlighted that treatment outcomes for macular edema are significantly worse with retinal laser photocoagulation alone, as compared with a combination treatment of photocoagulation and anti-VEGF therapy (3,4). The role of retinal photocoagulation in preventing disease progression in age-related macular degeneration (AMD) has also been limited, addressing the late “wet” stage of the disease only.
Despite the advent of various anti-VEGF therapies for patients with wet AMD, there are currently no treatments available to limit disease progression from the early stages of the disease. Consequently, patients with early AMD are recommended nutritional supplements, namely the formulation assessed in the Age-Related Eye Disease Study (AREDS), and advised to report to their physician promptly should their vision begin to deteriorate. Unsurprisingly, the search is on for a viable and effective solution for early-stage patients.
Compared with convention…
And this might be in the form of a non-thermal, three-nanosecond pulse retinal laser therapy, 2RT (Ellex, Adelaide, Australia). It has been labelled ‘Retinal Rejuvenation Therapy’ by the manufacturing company, which has high hopes for it to become the world’s first treatment for early AMD. Why is it different from conventional laser photocoagulation? The short green (532 nm) YAG laser pulse duration limits thermal spread outside the RPE and offers protection to the neural retina by removing the threat of thermal tissue damage, which is intrinsic to conventional laser treatment.
To put the three-nanosecond pulse of 2RT into perspective, we can compare its process and outcomes with those of conventional photocoagulation lasers. Conventional laser photocoagulation generates a thermal reaction because the laser energy is absorbed by the melanosomes in the pigment epithelium, heating the local region. This heat can be likened to the effects of poaching an egg, where the proteins are denatured instantly. On average, most clinicians set the power pulse duration at around 100 milliseconds when performing retinal photocoagulation. This timescale allows propagation of heat well beyond the pigment epithelium alone, potentially causing extensive damage to the surrounding retina, Bruch’s membrane and choroid. In contrast, the three-nanosecond pulse of 2RT, set at the therapeutic power range, localizes its injury insult to the RPE cells only, while causing no thermal damage to the underlying Bruch’s membrane and overlying photoreceptors (Figures 1 and 2) (5). With this approach it is possible to eliminate the thermal extension intrinsic to retinal photocoagulation while maintaining the efficacy of treatment with pinpoint accuracy. Essentially, it is a unique approach of lethally injuring a small, targeted monolayer: the RPE cells and allowing the adjacent RPE cells to subsequently recreate a normally functioning pigment epithelial layer. This triggering of a natural healing process is why it is called rejuvenation. It is comparable to skin rejuvenation, where cryotherapy causes damage to the more sensitive cells in the epidermis, while preserving the dermis. The epidermis is consequently repopulated from the surrounding dermis.
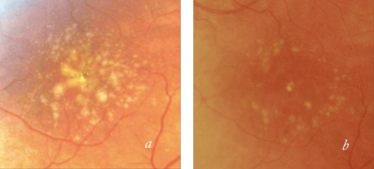
Figure 1. Drusen in a patient with AMD before (a) and after (b) 2RT 3 ns pulse laser therapy.
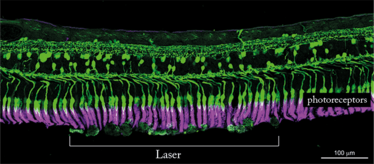
The 3 ns pulse laser energy delivered to the retina with the 2RT laser stimulates a biological healing response that improves the permeability of Bruch’s membrane and thereby restores the transport of fluid across it. As illustrated in the image above, this does not cause photoreceptor damage. By restoring metabolite flow to retinal cells, 2RT may also reduce the production of endothelial growth factors and other precursors of neovascularization.
Essentially, 2RT involves the repopulation of new cells from the adjacent healthy cells. The treatment effect stimulates a natural process of cell turnover and tissue restoration without causing any more injury than is needed. The effect of the three-nanosecond laser pulse should therefore be a healthy and rejuvenated cell layer surrounded by undamaged cells and membrane layers, which all contribute to preserving patients’ visual function.
What I have found, when comparing the microperimetry assessments of patients with early AMD before and after 2RT treatment, is that 2RT treatment was associated with large improvements in contrast sensitivity. By comparison, contrast sensitivity outcomes following standard thermal photocoagulation are weaker, owing to the blind spot caused by the thermal injury and the subsequent lack of functional improvement.
The 2RT effect can be adjusted according to the individual patient’s requirements. This is particularly important because the RPE pigment density increases closer to the fovea, resulting in a more intense reaction. The macular degeneration prevention strategy assessed in the 2RT pilot study achieved the effect from treatment near the major vascular arcades (6), in contrast to the exposure of conventional thermal photocoagulation for drusen to reduce neovascularization, which was nearer to the fovea. During the mid-1980s, studies had shown conventional lasers to induce the regression of drusen, but there appeared to be a high incidence of choroidal neovascularization, causing this method of treatment to be abandoned (7). Subsequent reviews of those results suggest that the rate of choroidal neovascularization was in keeping with the natural history cohort so that there was no greater risk with the laser, but no reduction in the risk either (5).
From experience
How is it used in practice? Based on my experience, 2RT takes between five and 10 minutes to perform. Following a local anesthetic and contact lens insertion, the laser power levels are brought up to threshold as defined by a slight opalescence at the RPE level. In the 2RT multi-center, randomized, sham-controlled LEAD trial (8), the treatment has been limited to six spots above and below the fovea, near to the major arcades. With this method, the therapeutic endpoint is a sub-threshold effect. After the application, the treated area is observed closely and it is important to wait a minute or two to assess the effect. The slight whitening or opacification of the epithelium which occurs over a few minutes is very different to the instant whitening observed in standard continuous wave retinal photocoagulation. Once the threshold has been determined, the power level is reduced, and you cannot observe any physical changes. Then, it is simply a matter of making 12 rapid applications of the laser treatment: six along the superior vascular arcade and six near the inferior one.
For the early AMD protocol, the ideal patient is defined as having drusen greater than 125 µm and functional disability. However, the candidate’s visual acuity should be 6/12 or better, before a more severe onset of visual loss occurs. Finally, ideal patients should not have dense cataracts, due to the difficulty of achieving a therapeutic endpoint.
Evaluating the impact
Much of our understanding of the outcomes of 2RT therapy can be gleaned from the 12- and 24-month pilot study data published by Robyn Guymer and colleagues, in which the changes in drusen area of 50 patients treated with 2RT were compared against a natural history cohort (5). Their results showed drusen reductions at 12 and 24 months of 40 percent and 35 percent, respectively, compared with 11 percent in the natural history cohort. An improvement in flicker threshold within the central three degrees at the third postoperative month was also observed in the 12-month data set. Seven of the 11 eyes at greatest risk of progression (where the flicker defect was greater than 15 dB) improved to the extent that they could be taken out of the high-risk category. Subsequent vast improvements in macular appearance and function were observed, which sustained themselves over 24 months. These findings provide circumstantial evidence that it is possible to delay the progression of AMD before it develops into its advanced “wet” form. Longer-term studies are required, however, to corroborate these observations. As part of a multicenter LEAD trial, we are now looking to evaluate the three-year outcomes, which will enable us to understand the longer-term effects of 2RT in the treatment of early AMD patients.
We have high hopes for a game-changer with this approach. Conventional AMD treatments, such as ongoing intraocular injections of anti-VEGF medications, address late-stage complications associated with AMD. In contrast, 2RT appears to offer the potential to apply treatment earlier in the disease process and to intervene at the level of the underlying pathology, preventing neovascularization and the progression to wet AMD.
Erica Fletcher and her colleagues at the University of Melbourne have demonstrated the effects of the three-nanosecond pulse 2RT in a mouse model (5), showing that the photoreceptors and retinal neurons are preserved and undamaged following 2RT. A healthy reaction is induced among the microglia, which sends down the pseudopodia to clear the debris, including the drusen-like material. Within an hour, the microglial changes that we can see are spectacular, demonstrating the efficacy of the healing response induced in the RPE. The process of improving hydraulic conductivity through the thinning of Bruch’s membrane is demonstrated over the entire retina rather than being confined to the lesion area on available animal models. Since a microglial reaction is induced, it is also upregulated in the fellow eye, so that drusen regression may also occur in the fellow untreated eye. The results show that while drusen was reduced in 44 percent of treated eyes, it was also reduced in 22 percent of untreated fellow eyes. Additionally, retinal structure was preserved over the treated area in comparison with the untreated area. It is important to note the widespread effect on the microglial activation and Bruch’s membrane structural changes despite the limited application areas.
These results have also been observed in human retina explants, where two enucleated eyes were examined following the application of 2RT at clinical and supra-threshold energy levels (5). Imaging which was conducted prior to and post collection of the enucleated eyes, demonstrated that there were enlarged RPE cells on the lesion boundary, with some cells extending into the lesion site. One month after treatment, enlarged RPE cells completely occupied the lesion site. In both cases, the application of 2RT had no effect on the structure of the retina. The endogenous retinal microglial processes extending through the outer nuclear layer towards the lesion site exhibited normal microglial responses in the retina.
In another context, 2RT has been shown to have a beneficial effect in reducing cystoid macula edema secondary to diabetic retinopathy. The treatment parameters are similar to those for early AMD, ideally with sub-threshold intensity, but these parameters are not as well defined at the moment. The potential advantage of 2RT is in the absence of photoreceptor damage or significant pigment epithelial destruction close to the fovea, where the fovea is an area that is not amenable to standard thermal laser treatment. Currently, anti-VEGF therapy is the only sight-preserving therapy available for perifoveal diabetic macula edema.
Delaying degeneration without photocoagulation
Naturally, AMD will continue to progress eventually, but this kind of rejuvenating treatment appears to delay the degeneration significantly. We still need to continue long-term studies to see the broader effects over time, but since we know that the treatment injures select, individual RPE cells while preserving surrounding cells and Bruch’s membrane, we can expect that the daughter cells will successfully slide in, being phenotypically and functionally normal. It is this sense of normality that sets 2RT apart from virtually every other form of retinal laser treatment, in my opinion, where the surrounding tissue is damaged by heat in the process. With 2RT we have a procedure that is minimally invasive, can be performed in-office within minutes, and with almost no risk of infection. It can therefore potentially revolutionize AMD management, particularly because it allows us to treat patients in the early stages of AMD rather than waiting until the disease has progressed into its “wet” form.
Wilson Heriot is a principal at Eye Surgery Associates, Melbourne, Australia, and chair of the Oceania Retina Association and board a member of the Macular Degeneration Foundation.
- M Shimura, et al., “Visual dysfunction after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision”, Am J Ophthalmol, 140, 8–15 (2005). PMID: 15939392.
- KE Higgins, et al., “Temporary loss of foveal contrast sensitivity associated with panretinal photocoagulation”, Arch Ophthalmol, 104, 997–1003 (1986). PMID: 3729795.
- MJ Elman, et al., “Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema,” Ophthalmology, 117, 1064–1077.e35 (2010). PMID: 20427088.
- MJ Elman, et al., “Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema,” Ophthalmology, 118, 609–614 (2011). PMID: 21459214.
- AI Jobling, et al., “Nanosecond laser therapy reverses pathologic and molecular changes in age-related macular degeneration without retinal damage”, FASEB J, 29, 696–710 (2015). PMID: 25392267.
- RH Guymer, et al., “Nanosecond-laser application in intermediate AMD: 12-month results of fundus appearance and macular function”, Clin Experiment Ophthalmol, 42, 466–479 (2014). PMID: 24118741.
- Choroidal Neovascularization Prevention Trial Research Group, “Lasertreatment in eyes with large drusen: short-term effects seen in pilot randomized clinical trial”, Ophthalmology.105, 11–23 (1998). PMID: 9442774.
- Clinicaltrials.gov, “Laser Intervention in Early Age-Related Macular Degeneration Study (LEAD)”, NCT01790802, clinicaltrials.gov/ct2/show/NCT01790802 (2015). Accessed August 20, 2015.
Wilson Heriot is a principal at Eye Surgery Associates, Melbourne, Australia, chair of the Oceania Retina Association and a board member of the Macular Degeneration Foundation. Following his training in Melbourne, he spent two years in New York, USA, researching pigment epithelial phototoxic injury, and initiation of choroidal neovascularization. His current research focus is treatment advances in AMD.